Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zuzanna Pelc and Version 2 by Peter Tang.
Breast implantation (BI) is the most common plastic surgery worldwide performed among women. Generally, BI is performed both in aesthetic and oncoplastic procedures.
- breast implantation
- breast implant illness
- breast implant-associated anaplastic large cell lymphoma
1. Silicone Breast Implants Crisis
In the early 1990s [1][11], first reports about the potential link between silicone breast implants and autoimmune or rheumatic diseases led to doubts concerning the safety of silicone implants. As a result, the FDA precluded third-generation silicone implants [2][3][6,12] without any specific reason [4][13]. Consequently, silicone breast implants were unapproachable [4][13]. For two years saline-filled implants were the only method accepted by the FDA for aesthetic breast augmentation. Additionally, the consensus was limited to a narrow group of procedures: temporary expanders awaiting for permanent reconstructive surgery, reconstructive surgery during mastectomy, and ruptured silicone gel breast implants pending replacement [5][6][5,8]. The decision of the FDA to suspend the use of silicone implants and requesting manufacturers to provide additional data on both the safety and effectiveness of the 3rd generation silicone implants resulted in the creation of the 4th and 5th generation silicone implants [2][3][6,12]. These implants have been designed following the stringent guidelines of the American Society for Testing Methodology (ASTM) and the FDA [2][3][6,12]. New generated implants were characterized with better quality and a more comprehensive range of surface textures and shapes due to new parameters of the implant shell’s thickness and the cohesiveness of the gel filling.
In consequence, after 14 years of suspension, silicone breast implants were reintroduced in the United States [7][9]. The decision of the FDA was supported by many studies proving silicone breast implants safety and its role in improving the quality of patients’ life. Therefore, with the benefit of hindsight, it is clear that the suspension of silicone breast implants has brought many advantages. As a result, a few studies have been performed to verify the effects of silicone implants rupture, their relationship to autoimmune diseases, or patient satisfaction after breast augmentation surgery [7][8][9,14]. Silicone implants of the 4th and 5th generation, created due to the withdrawal of 3rd generation silicone implants, together with saline-filled implants, are currently used [9][10]. The timeline of breast augmentation history is shown in Figure 1.
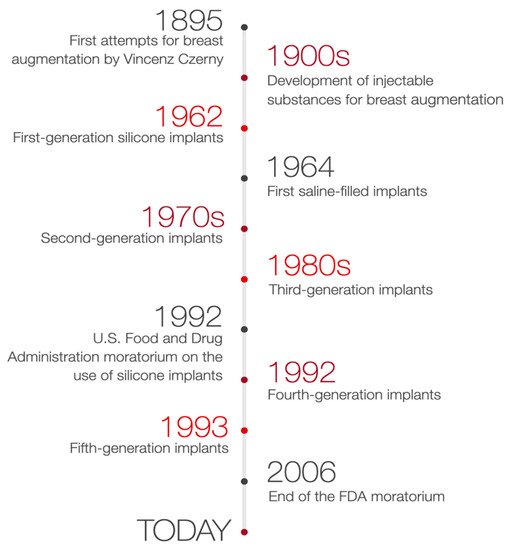
Figure 1.
Timeline of breast augmentation history.
2. Characteristics of Breast Implants
Breast implants are applied in plastic surgery for breast augmentation or reconstruction surgery among patients undergoing a partial or complete mastectomy due to oncological or aesthetic reasons [10][15]. There are two primary breast implants: saline- and silicone gel-filled implants [11][12][13][16,17,18] composed of a silicone elastomer shell with a smooth or textured surface [11][12][16,17]. To select the implant most suited to the patient’s needs, the implants height, width, and projection can be adjusted. Nevertheless, it is recommended that surgeons operate with both round and anatomical implants and adapt the specific type of implant individually. The choice of a particular type of implant may vary depending on the situation. According to the FDA guidelines, a patient must be at least 18 years old to undergo saline-filled breast implant surgery and 22 years old to be qualified for silicone gel-filled implants [10][15]. The generations of silicone breast implants are shown in Figure 2.
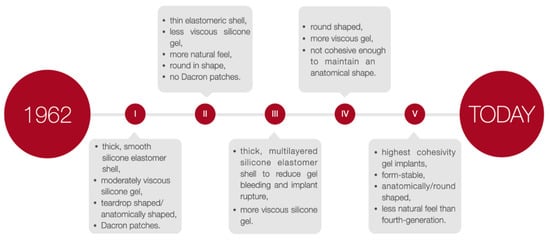
Figure 2.
The generations of silicone breast implants.
3. Breast Augmentation Surgery—Preoperative Management
Breast augmentation surgery for 45 years was considered an isolated surgical procedure, consisting of a breast implant placement in a pocket created by a plastic surgeon. The procedure has been redefined and extended beyond the actual placement of a breast implant [13][18]. It occurred that breast augmentation surgery is more complicated than initially thought. Both short and long-term outcomes of the procedure are determined by various alternatives and differences, which surgeons must consider during the planning and performing breast augmentation surgeries and further complications or risk of reoperation [14][21]. Therefore, proper preoperative decisions and aspects of surgical technique emerged to be equally important. One of the controversies is the manner of one or two-stage surgery [15][22]. Traditional and safe two-stage reconstruction relies on a tissue expander/submuscular or prepectoral plane insertion with the following exchange for a permanent implant. In contrast, a permanent implant is placed immediately after mastectomy during a single-stage reconstruction, known also as direct-to-implant procedures [15][22]. Improvement of surgery results and performance efficiency and reduction of reoperations rate can be obtained by integrating preoperative quantitative tissue assessment with five critical decisions in breast augmentation surgery [14][21]. Consequently, while making preoperative decisions, surgeons must consider five crucial areas of planning breast augmentation surgery. They are ordered by importance and includes optimal soft tissue coverage/breast implant pocket position, implant volume (weight), type, size and dimensions of the breast implant, proper location for the inframammary fold, and the incision place [14][21]. The following element of preoperative planning includes patient education [13][16][18,23]. A complete understanding of the patient’s entire breast augmentation process, choice of implant’s type, and shape are crucial aspects that should be clarified before intervention. The educational features should raise the patient’s awareness of the existing limitations of a procedure and the possibility of complications, along with the specific plan for the treatment after breast implantation.
4. Incision Site and Implant Placement
The incision before BI may be located in the inframammary, periareolar and transaxillary area [17][24]. Its selection depends on the patient’s individual preferences and potential benefits and risk factors [18][25]. Inframammary and periareolar incisions are the most popular choices [13][17][18][18,24,25]. The inframammary technique provides easy access, greater control through the precision of dissection and hemostasis of the pocket. Additionally, almost any type and size of breast implant might be used and damaging the breast tissue could be avoided. However, this type of incision is a potential risk factor of an unaesthetic scar formation. The periareolar incision allows proper access to the breast, and the formed scar mostly remains invisible. Nevertheless, poor scar formation, the higher risk of capsular contracture or an enhancement of the sensitivity of the nipple-areola complex are the potential complications. The undeniable advantage of the transaxillary incision is the lack of any scar in the breast area. Although, this method includes less control over the release of the pectoral muscle if an endoscope is not available and a lack of the possibility of dual-plane dissection.
Another crucial step in BI surgery is implant placement. Usually, implants are placed either submuscularly—under the pectoral muscle or subglandularly—above the pectoral muscle but below the breast glandular parenchyma [19][26]. Submuscular placement of a breast implant is associated with a lower risk of capsule contracture and more accessible mammography imaging [5]. Due to the relative ease of surgery and the ability to achieve the desired cosmetic effect, the subglandular location remains a frequent choice. The following option is a subfascial placement consisting of placing the breast implant as an alternative to the submuscle location and represents a compromise between the submuscular and subglandular implantation [20][27]. Despite satisfactory results and rapidly gained popularity, the subfascial implant placement technique remains controversial. Another procedure, the dual-plane technique, remains a modification of the submuscle placement of the breast implant, which creates a plane of surgical dissection between the fascia of the pectoralis major and subglandular tissue muscle partially cover the surface of an implant [21][28]. According to the consensus of experts in breast augmentation from Australia and New Zealand, the dual-plane technique was the most frequently used [19][26]. Breast implants placement locations are shown in Figure 3.
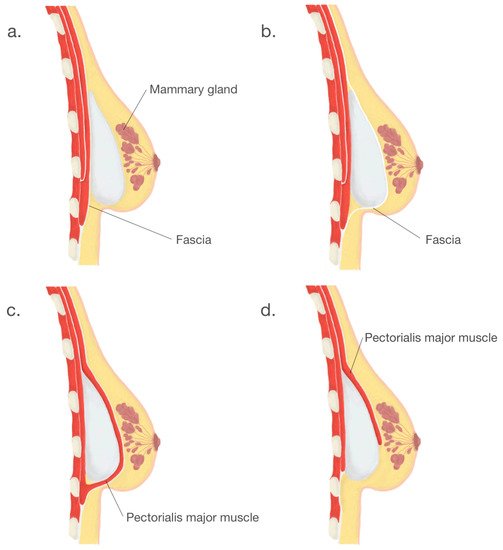
Figure 3.
Breast implants placement locations: (
a
). subglandular, (
b
). subfascial, (
c
). submuscular, (
d
). dual—plane.
5. Implant-Related Complications
Two common and acknowledged complications of breast augmentation surgery are implant rupture and capsular contracture [22][29]. The mechanisms behind the rupture of breast implants have been thoroughly investigated.25 Damage of the implant shell by surgical instruments, a flaw of the fold, swelling of the implant surface or a manufacturing defect are among the most common [23][24][25][30,31,32]. Risk factors for rupture of breast implants also include excessive forces on the chest, e.g., during closed capsulotomy, due to an injury with seat belts or blunt trauma, and the result of breast compression after mammography or severe capsular contracture. American manufacturers of breast implants regularly analyze the leading causes of ruptures of implants. Handel et al. analyzed data from Mentor and Allergan about breast implant fractures and concluded that approximately 51–64% of the implants were recorded as damaged by surgical instruments [24][31]. Breast implant ruptures are classified as intra-capsular and extra-capsular [26][33]. Intra-capsular rupture of a breast implant is complicated to identify with routine imaging methods such as mammography or ultrasound [27][34]. Therefore, it is usually detected during surgery.
Despite extensive research, the mechanism behind the contracture of the implant capsule remains unclear and not fully understood [28][29][38,39]. Bachour et al. suggest that the incidence of capsular contracture increases due to the gel leakage during implant rupture [30][40].
A greater risk of contracture of the implant capsule is associated with inserting a breast implant with a smooth surface and subglandular positioning. Various scales have been proposed to classify a grade of contracture of the breast implant capsule, e.g., Baker and Wilflingseder classifications [31][42], as shown in Table 1.
Table 1.
Clinical classification (Baker score) and histological classification (Wilflingseder score) of capsular contracture.
Grade | Baker | Wilflingseder | ||||||
|---|---|---|---|---|---|---|---|---|
I | Implant shell not palpable and not visible | Thin and uncontracted capsule | ||||||
II | Implant shell slightly firm, but not visible | “Constrictive fibrosis”, no giant cells | ||||||
III | Implant shell clearly firm and implant visible | “Constrictive fibrosis”, giant cells present | ||||||
IV | Implant shell very firm, implant dislocation and deformation | Inflammatory cells, foreign body granulomas, neovascularization, possible neuromas |
6. Breast Implant-Associated Anaplastic Large Cell Lymphoma
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is an uncommon non-Hodgkin’s T-cell lymphoma [32][33][45,46], characterized by a monoclonal population of CD30+ large anaplastic cells, negative anaplastic lymphoma kinase and variable expression of lymphocyte T and EMA markers [21][34][28,47]. The first case of BIA-ALCL described in 1997 by Keech and Creech was reported in a patient with saline implants [35][48]. In 2016, the World Health Organization acknowledged anaplastic large cell lymphoma (ALCL) associated with breast implants as a new type of malignancy [36][37][49,50], whereas In 2017, BIA-ALCL was included in the classification of haemato-lymphoid neoplasms [37][50]. Until now, the American Society of Plastic Surgeons Patient Registry has already collected 518 cases of BIA-ALCL from 25 countries [33][46], with the incidence defined at approximately one to three per million per year [33][38][46,51]. Nevertheless, the incidence of this condition is more prevalent than expected, as shown in recent Review by Santanelli di Pompeo et al. [37][50] Although most cases are indolent, the clinical features may include redness, breast pain and swelling, asymmetry, palpable tumour, seroma or ulceration [39][40][41][52,53,54]. Diagnosis is verified by cytological, immunohistochemical and immunophenotypic assessment of the aspirated peri-implant fluid [41][54]. Reports indicate that the ALCL affects patients with silicone and saline breast implants with a textured surface or tissue expanders [17][33][42][24,46,55]. Nearly all cases of ALCL are associated with textured surface breast implants [17][39][43][24,52,56]. However, this data was not confirmed in a meta-analysis conducted by Ramos-Gallardo et al. [21][28]. An increasing number of studies suggest a multifactorial cause of the development of BIA-ALCL: bacterial component, implant surface texture, genetic factors, and mechanical friction [32][43][44][45,56,57]. The presence of bacteria may reflect an opportunistic infection. The larger the surface area of the implant shell, the greater the probability of bacterial growth [32][33][39][45,46,52]. Jones et al. divided the textured implants into macro- and microtextured by considering the morphology of the outer shell of the textured implants [33][46]. They established a four-grade classification, with studies showing that the risk of developing ALCL is significantly higher for grades 3 and 4.
7. Breast Implant Illness
When the BI gained immense popularity, controversy regarding augmentation has also started to emerge [45][46][63,64]. The complications after surgery, such as the contracture of the implant capsule, rupture of the implant or its incorrect position, and possible relationships of inserted implants with autoimmune diseases, started to raise concerns. Currently, there are no studies that could confirm this theory [45][47][48][63,65,66]. However, a constantly increasing percentage of patients report symptoms negatively affecting their mental and physical functioning [41][54]. Chronic pain and fatigue, memory and concentration disorders, panic attacks, and depression [46][64]. Symptoms of Breast Implant Illness (BII) also include joints and muscles pain, mouth and eyes dryness, alopecia, skin lesions and Raynaud’s syndrome [47][65]. A significant group of patients manifesting these symptoms initiated linking them with breast implants, which eventually led to the creation of the BII term [1][11]. The history of this phrase dates back to the 1960s, when the compilation of symptoms occurring among patients for the first time was defined as a human adjuvant disease [1][11]. The term was then used to describe a potential relationship between injecting paraffin, petroleum products and silicone to patients and developing autoimmune connective tissue disorders.
Currently, the term BII is used to describe a diverse and complex group of over 100 symptoms, focused on the following systems: the central nervous system, the musculoskeletal system, the immune system, the urogenital system, circulatory-respiratory system, the skin and its appendages and the psychological sphere [45][49][63,67]. The mechanism of development of BII is still unknown, even though research on possible associations of silicone breast implants with systemic diseases has been ongoing for over 40 years [48][50][66,68]. One of the theories explaining the occurrence of these non-specific symptoms is the development of an inflammatory or autoimmune reaction induced by the presence of a stimulant, in this case, silicone contained in breast implants [47][65]. Other theories suggest the pathophysiological basis of BII within social and psychological factors [45][63]. Unfortunately, the number of studies on BII is very limited. Currently, there are no diagnostic tests that would facilitate an adequate diagnosis, and in addition, there are no recommendations that would differentiate BII from other diseases [45][48][63,66]. Furthermore, BII is currently not considered a disease and has not been included by the World Health Organization in the latest ICD-11 classification. Nonetheless, Rohrich et al. indicate even more specific term for the syndrome called “silicone implant illness” [51][69]. In contrary, Lee et al. suggest BII symptoms seem not to correlate with any particular implant type, surface, or filling [49][67].
