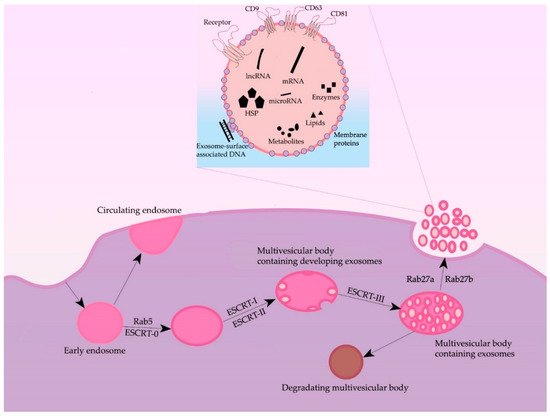
2. Formation and Secretion of Exosomes
Formation of exosomes—lipidic bilayer vesicles (30–150 nm) that carry CD9, CD63, and CD81 tetraspanins on their surface—begins with the invagination of plasmalemma, forming a clathrin-coated vesicle—early endosome (
Figure 1). The early endosome can either be fused with the membrane back, in which case it is called a “circulating endosome”, or, after loading by nucleic acids, proteins and lipids, transforming into multiple intraluminal vesicles; intraluminal vesicles mature to the multivesicular bodies (MVBs). The main regulator of the transition from the early endosome to the multivesicular body is the small GTPase Rab5, in cooperation with the effector VPS34/p150
[4][15]. Once bound to Rab5, the effector proteins phosphoinositol-3-kinase, early endosomal antigen 1, and rabenzin-5 become active, which stabilizes the active form of Rab5, promoting further recognition by the FYVE domain that is part of the endosomal sorting complexes required for transport (ESCRT complex). The ESCRT-0 complex recognizes ubiquitinated proteins using the HRS and STAM1/2 heterodimer
[5][16]. ESCRT-0 involves ESCRT-I and ESCRT-II complexes, which trigger the formation of exosomes in the membrane of the multivesicular body. Next, the ESCRT-III complex is involved, promoting detachment from the membrane and release of vesicles inside the multivesicular body
[6][17]. The multivesicular body can then either lose its ubiquitin tag by means of a group of proteins or fuse with the lysosome and undergo degradation. Then, by the proteins Rab27A and Rab27B, the multivesicular body moves to the periphery of the cell and fuses to the membrane
[7][18]. Moreover, the inactivation of Rab27A and Rab27B reduces exosome secretion only by half, suggesting the existence of alternative pathways for secretion. The effect of Rab5A, Rab9A, and Rab2 proteins on exosome secretion has also been shown
[7][18] (
Figure 1). Furthermore, both normal and tumor cells have been shown to increase exosome secretion under unfavorable conditions (hypoxia, heat shock, etc.)
[8][19].
Figure 1. General scheme of processes of formation and secretion of exosomes and their main regulators.
Some researchers also note an ESCRT-independent pathway of exosome formation
[9][20]. According to some data, tetraspanins are important players in the ESCRT-independent pathway: the expression of CD9 and CD82 increased the secretion of exosomes from HEK293 cells
[10][21]; Tspan8 tetraspanin expression did not affect the total number of secreted exosomes but changed their mRNA and protein composition
[11][22]. CD63 is also involved in exosome biogenesis: a recent study showed that CRISPR/Cas9 knockout of CD63 led to a decrease in vesicle secretion
[12][23]. Another protein thought to play an important role in exosome formation is the lysosome/late endosome small integral membrane protein (SIMPLE). Transfection of COS cells with this protein resulted in increased secretion of exosomes, and its mutations, in disruption of medulloblastoma formation.
The secretion of exosomes into the extracellular space is caused by the fusion of the MVBs with the plasmalemma. The fusion process begins with the synaptotagmin protein interacting with calcium receptors, causing the multivesicular body binding to the trans-SNARE complex, followed by exosomes secretion into the extracellular environment
[13][24].
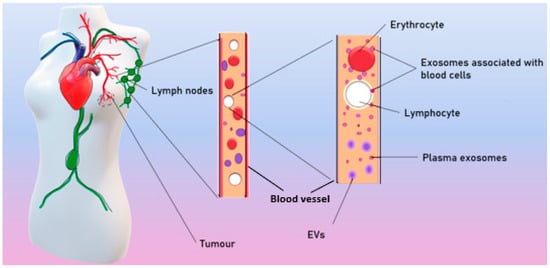
Exosomes are ordinarily released by different cell types and can both locally affect nearby cells or distally affect cells, spreading to the tissues with the blood and lymph. As a result, exosomes are found in all biological fluids of healthy individuals
[14][25]. In patients with BC, tumor exosomes spread in the organism and are found in the blood
[15][26], ascites
[16][27], tears
[17][28], and breast milk
[18][29] (
Figure 2)
.
Figure 2. Spreading of tumor exosomes in organism.
According to different published sources, it is known that there are from 50 to 300 million exosomes per 1 mL of blood in healthy donors
[15][26]. Theoretically, each exosome contains lipids, no more than 100 proteins, and no more than 1000 microRNA
[19][30]. Moreover, a number of studies have shown that exosomes in the blood circulate in a free form but are also associated with surface of blood cells (csbExos)
[15][20][13,26], allowing them to move through the organism over long distances. It has also been shown that csbExos contain twice as much RNA as exosomes circulating in blood plasma
[20][13], which may indirectly indicate a significant role of exosomes associated with blood cells in tumor progression.
Several mechanisms have been suggested for transfer of exosomal cargo to recipient cells. There are (i) fusion of exosomes with the cellular membrane, leading to the release of exosomal content into the cytoplasm of the recipient cell; (ii) juxtracrine signaling through ligand–receptor interactions; (iii) endocytosis by phagocytosis
[21][31].
3. Role of Exosomes in Breast Tumor Progression
Although malignant cells themselves are the major source of tumorigenesis, their interactions with the tumor microenvironment are critical for the progression from a single tumor mass to distant metastases. Derived from cancer cells, exosomes mediate EMT, malignant cell proliferation and motility, metastatic processes, angiogenesis stimulation, and immune system repression.
3.1. Epithelial–Mesenchymal Transition
EMT is a necessary precondition for metastasis; it is usually followed by the transition of epithelial to mesenchymal cells. Such a process is crucial for the metastasis process so that epithelial cells could acquire the ability to move through blood vessels
[22][68]. The EMT program is usually activated during metastasis and tumor invasion; hence, the genetic changes and molecular mechanisms by which cancer cells acquire invasiveness and their subsequent metastatic ability have been areas of intensive research.
Tumor-derived exosomes also carry some oncogenic microRNAs, such as miR-23a, miR-5100, miR-19b-3p, and miR-21, which control the EMT process
[23][69]. The levels of expression of such microRNAs in blood could be the basis for various exosomes-based diagnostics
[23][69]. For example, discussed above as metastatic, causing proliferation, and increasing migration, exosome-encapsulated miR-1910-3 p is a biomarker of BC that circulates in the blood
[24][70].
MiR-34a, which is a p53 transcription target, is reported to inhibit the aggressiveness of BC cells by inhibiting EMT and zinc finger transcription inhibitor Snail
[25][71]. The balance between the expression levels of the miR-22 and miR-200 families also regulates the EMT phenotype in BC, so these microRNAs are considered an important factor in controlling the first steps of metastasis
[26][27][72,73].
Cancer-associated fibroblast (CAF)-derived exosomes carrying miR-181d-5p are suggested to enhance proliferation, invasion, migration, and EMT and suppress apoptosis of BC cells through regulation of
CDX2 and
HOXA5 genes. It was announced that overexpression of HOXA5 inhibits proliferation, migration, invasion, and EMT and induces apoptosis of MCF-7 cells. Transcription factor CDX2 binds to the Hoxa5 promoter and promotes HOXA5 expression, while miR-181d-5p mimics CDX2 to reduce expression
[28][74].
The EMT phenotype can also be induced by miR-103/107 in BC cells. It was found that miR-103/107 weakened microRNA biosynthesis by targeting the gene encoding Dicer, leading to the total downregulation of microRNAs, including the miR-200 family, as well as the further development of EMT and the metastatic phenotype of epithelial tumor cells
[29][75].
3.2. Proliferation
Reaching the Hayflick limit, most of the cells enter a process of programmed self-liquidation, called apoptosis. Malignant tumor cells are able to avoid apoptosis by increasing the expression level of many antiapoptotic genes of the
BCL2 family, as well as by inducing oncogenic mutations in
p53 and hyperexpression of apoptosis inhibitor proteins. As a result, BC cells go through the stages of the cell cycle faster and the level of cell proliferation increases
[30][76]. Cyclins and cyclin-dependent kinases have also been shown to have a regulatory role in accelerating the cell cycle as the expression level of these kinases affects changes in the cell cycle
[31][77].
The results of in vitro uptake of exosomes derived from murine BC cell line 4T1 showed equal efficiency for CD133+ and CD133- cell lines
[32][78]. CD133+ is a marker of cancer stem cells, and its expression is associated with a poor prognosis and chemoresistance in some types of solid tumors, including BC. Meanwhile, in vitro analysis of cell proliferation showed that exosomes from 4T1 cells significantly increased proliferation of CD133+ cells but had no effect on CD133- cells. In addition, the level of apoptosis in CD133-positive cells after treatment by doxorubicin (an apoptosis-inducing drug) was significantly suppressed by exosomes originating from 4T1, whereas, in CD133-negative cells, it did not occur. The results allowed the authors to suggest that exosomes from tumor cells may function as pro-tumor factors, contributing to active proliferation and suppression of apoptosis of CD133+ tumor stem cells
[32][78].
One of the recent research studies has shown that exosomal miR-500a-5p derived by cancer-associated fibroblast significantly promotes proliferation in MDA-MB-231 and MCF-7 BC cell lines via targeting the USP28 protein
[33][79]. Furthermore, the level of MiR-1910-3p is significantly higher in BC cell lines MDA-MB-231 and MCF-7 compared to pseudonormal epitheliocytes line MCF-10A; furthermore, it was shown that miR-1910-3p promotes cell proliferation in vivo by activating the NF-kB signal pathway
[22][68].
The study of blood exosomes revealed that the addition of total blood exosomes (from the plasma and from blood cell surface) from BC patients led to a statistically significant increase in the mitotic event number of MCF-10A cells in contrast to plasma exosomes. These results indirectly indicate that csbExos are responsible for the stimulation of cell proliferation. It was an unexpected finding that total blood exosomes of healthy females stimulated the proliferation of MCF10A too
[34]. Since most exosomes in the blood of cancer patients are of non-tumor origin, it is likely that, without attracting attention to them, exosomes from normal cells play a significant role in tumor dissemination.
3.3. Cell Motility
In addition to the effect on cell proliferation, tumor-derived exosomes can also change the ability of recipient cells to migrate. For example, miR-9, delivered by exosomes from cancer cells to fibroblasts, leads to their transformation into tumor-associated fibroblasts with increased cellular motility
[35][80].
Further, miR-135a has been reported to be highly expressed in metastatic breast tumors. It was found that the expression of miR-135a is necessary for the migration and invasion of BC cells, but not for their proliferation.
HOXA10 encodes a transcription factor necessary for embryonic development and is a metastatic inhibitor in BC. It has been shown to be a direct target of miR-135a in BC cells. The study has truly demonstrated that miR-135a inhibits HOXA10 expression at both mRNA and protein levels, and its ability to promote cell migration and invasion is partially reversed by HOXA10 overexpression
[36][81].
Further, miR-130 delivered into macrophages by exosomal transport has been reposted to cause M1 polarization (macrophage repolarization from M1 to M2 phenotype). It was also shown that the phagocytosis ability of macrophages enhanced after being treated by microRNA-loaded exosomes. Thus, migration and invasion assays demonstrated reduced ability of 4T1 BC cells for migration and invasion after macrophages reprogramming
[37][82].
It is shown that miR-148a and miR-16 upregulate MDA-231 breast cancer cells’ motility via targeting
CCNE1,
Twist1, and
Wnt10b [38][83]. Furthermore, MiR-7641 is upregulated in highly metastatic BC cell line MDA-MB-231 in comparison to non-metastatic MCF-7 cells and is shown to upregulate cell motility
[39][84].
The study of the effect of blood exosomes on cell motility showed that the addition of exosomes from the total blood of healthy females or from the plasma and total blood of BC patients resulted in a significant increase in the motile MCF-10A cell number compared to the negative control. MicroRNA analysis associated with cell motility revealed the increased level of miR-92a in plasma exosomes and level miR-25-3p in total blood exosomes of BC patients compared to healthy females
[34].
Adipocyte-derived exosomes from cell line 3T-L1A were shown to increase BC cell motility in cell lines MCF-7 and MDA-MB-231 via increasing the level of HIV-1α
[40][85].
3.4. Metastasis
The mortality rate from BC is determined by the development of distant metastases to the lung, brain, bones, and liver. Predicting the site of probable metastasis is important for determining the therapeutic algorithm that could prevent the spread of tumor cells. The capacity of exosomes to affect the invasiveness and, consequently, metastasis of cancer tumors has been shown both in vitro and in vivo
[41][86]. Moreover, exosome cargo impacts tumor niche establishment and regulates the tropism of metastasis
[42][87].
Analysis of MCF-7 cell exosome proteins (characterized by low metastatic potential) showed increased content of tetraspanin superfamily proteins (Tetraspanin-14, CD9, CD63, and CD81, which enhance cell adhesion and decrease the propensity for migration and metastasis, and, in exosomes of MDA-MB-231 line (characterized by high metastatic potential), proteins enhancing cell motility (Vimentin, Galectin-3-binding protein, Annexin A1, Plectin, Protein CYR61, EGF-like repeat, and discolding I-like domain containing protein, Filamin-D, Protein-glutamine gamma-glutamyltransferase 2) were hyperrepresented
[43][88]. Exosomes have also been shown to carry survivin, enhancing the expression of SOD1, which controls differentiation of fibroblastic cells to myofibroblasts, disrupting fibroblast adhesion, allowing metastasizing tumor cells to invade new sites, and increasing proliferation of BC cells
[44][89]. Exosomal aspartate-β-hydroxylase is also involved in BC metastasis: it has been shown that the enzyme triggers the Notch signaling pathway that induces exosome secretion and increases cell aggressiveness.
Molecular insight into exosome cargo has disclosed the pivotal roles of microRNAs in BC metastasis; for example, it has been widely demonstrated that exosomes secreted by BC cells promote CAFs activation by the miR-146a/
TXNIP axis to start the
Wnt pathway, which, in turn, increases the metastasis and invasiveness of BC cells
[45][90]. Further, miRNA-9, carried by exosomes, is shown to be a pro-metastatic microRNA and is abundant in several BC cell lines. Additionally, it is able to induce switches in the cancer phenotype in normal fibroblasts and thus promotes tumorigenesis. However, some studies suggest that miR-9 also acts as anti-oncogene in BC proliferation; that is, it inhibits the occurrence of BC at an early stage of the disease
[46][91]. For instance, miR-10 was first determined as a major regulator of metastasis in BC. The investigations showed that the expression levels of miR-10b were much higher in metastatic than non-metastatic BC cell lines. Ectopic expression of miR-10b in non-metastatic BC cell lines induces upregulation of Ras homologous gene family members by direct targeting of the gene encoding homeobox D10, which leads to the promotion of invasiveness and metastasis
[46][91].
Further, miR-19a, which belongs to the miR-17-92 cluster, is reported to have a tumor-promoting role in multiple types of cancers. It is also known to target PTEN and is predicted to target ER. MiR-19a expression is reported to positively correlate with osteolytic bone metastasis in vivo, strongly suggesting a role of miR-19a as a key modulator of tumor microenvironment in the process of bone metastasis of BC. Therefore, exosomal miR-19a mediates cell–cell communication between BC cells, promoting the vicious cycle of bone metastasis in ER + BC
[47][92].
Further, miR-21 and miR-200 have been shown to be differentially expressed in BC cells’ exosomes. Some studies performed using exosomes isolated from tear liquid have suggested that the amounts of miR-21, and miR-200c were significantly higher in tear exosomes isolated from patients with metastatic BC than those of healthy controls. Those data assure tears as an alternative biological liquid that requires less purification
[17][28].
BC cells produce exosomes carrying miR-122 that is shown to stimulate metastasis by forming the pre-metastatic niche. In this case, these exosomes block glucose uptake by pre-metastatic niche cells and breaking the energy metabolism, promoting cancer cells to progress
[48][93].
Further, miR-1910-3p contained in exosomes is shown to activate proliferation, migration, and autophagy. Meanwhile, it was reported to inhibit apoptosis in TNBC and ER- and PR-positive BC cells. Studies suggested that miR-1910-3p, carried by TNBC and ER- and PR-positive BC cells inhibits MTMR3, activating the NF-κB signaling pathway
[22][68].
Furthermore, it was shown that breast cancer cells of MDA-MB-231 treated by exosomes from the 3T-L1A cell line show increased metastatic potential during in vivo experiments on mice
[40][85].
3.5. Angiogenesis
Exosomes secreted by tumor cells are involved in active angiogenesis, caused by hypoxia. It was shown that BC cell lines MCF-7 and MDA-MB-231 show increased levels of secreted exosomes in a 0.1% O
2 environment compared to a 1% O
2 environment
[49][94]. Particularly, exosomes secreted by BC cells stimulate transformation of mesenchymal stem cells to myofibroblasts through the SMAD-mediated pathway (transforming growth factor signaling pathway). Myofibroblasts are among the core cells in the tumor, being involved in the processes of vascular reorganization
[50][95]. Furthermore, exosomes secreted by BC tumor cells are known to induce fibroblast activation, which stimulates
[51][96]. Assessment of the angiogenic potential of total blood exosomes and plasma exosomes of BC patients showed a comparable effect: they stimulated the formation of capillary-like structures from human umbilical vein endothelial cells, although they had elevated levels of various angiogenesis-stimulating microRNAs—miR-92a and miR-25-3p
[34].
Some members of the miR-17-92 cluster are able to enhance pro-angiogenic effects by targeting thrombospondin 1, VEGFA, and TIMP. Further, miR-20a, as a member of the miR-17-92 cluster, was shown to be able to induce increased vascular mesh and glomeruloid microvascular proliferation in BC, probably relating to overexpressed VEGFA
[52][97]. VEGF has been shown to be implicated in regulating various microRNAs, e.g., c-Myc oncogenic miR-17-92 cluster, and it is also capable of rescuing the promoted thrombospondin-1 level, loss of endothelial cell proliferation, and development of morphological properties associated with the loss of Dicer. It is noteworthy that the pleiotropic role of miR-17-92 can be considered for breast carcinoma, which can affect angiogenesis and/or the metastatic phenotype
[52][97] depending on the regulation of both ER pathways and tumor suppressors, as well as extracellular matrix changes
[53][98].
An anti-ancogenic effect of some exosomal microRNAs is being widely studied as microRNAs carried by exosomes are supposed to be relevant for a targeted therapy in the future. For example, exosomes from BC cells with a lower level of Ca
2+ were shown to contain more miR-145, targeting to IRS 1 to demonstrate an anti-angiogenic effect. Therefore, the reduction in the Ca
2+ level in cancer cells is possibly able to contribute to antiangiogenic tumor therapy
[54][99].
3.6. Immunosuppression
Immune escape of BC cells is important in the pathogenesis of BC.
Stress of the endoplasmic reticulum can be caused by a violation of protein homeostasis. MicroRNA-mediated mRNA translation inhibition has been extensively studied in the regulation of endoplasmic reticulum stress in various types of cancer. In particular, in BC, exosomal miR-27a-3p increased PD-L1 expression through the
MAGI2/PTEN/PI3K axis, thereby contributing to immune response evasion
[55][100]. Moreover, exosomes derived from cancer-associated fibroblasts were found to carry miR-92, which also upregulated PD-L1 expression in BC cells
[56][101].
Tumor exosomes are able to interact with macrophages, changing their phenotype
[57][102]. In particular, it was shown that exosomes secreted by BC cells carry glycoprotein 130 (gp130), which, together with miR-301a-3p, causes
STAT3 signaling pathway activation, resulting in an increase in reactive oxygen species concentration in macrophages
[53][58][98,103]. Moreover, after interaction with tumor exosomes, the secretion of interleukin 6, TNFα, and CCL2 in macrophages increases. It was also shown that, upon inhibition of gp130, macrophages differentiate via its normal pathway. Macrophage uptake of tumor exosomes results in a loss of HLA-DR antigen and increased CD14 expression, i.e., change in the phenotype to an immunosuppressive one
[59][104].
Besides macrophages, tumor exosomes can induce apoptosis among activated T-cells carrying the CD8 receptor
[60][105]. This effect may be related to the expression of MHC class I on the exosomal membrane surface, which triggers T-lymphocyte apoptosis via the FasL/Fas signaling pathway
[61][106]. For exosomes secreted by tumors, the ability to induce proliferation of suppressor cells of myeloid origin was also demonstrated, increasing its immunosuppressive abilities due to production of a variety of factors causing T-cell apoptosis
[62][107]. Protein programmed death factor ligand 1 (PD-L1) plays an important role in suppressing the immune response: it binds to the receptor on the surface of T-cells PD-1, resulting in a suppression of proliferation and cytokine release. PD-L1 has been detected in exosomes, in co-detection with Galectin 9, which, by interacting with the Tim-3 protein, which can also be transported by exosomes, also suppresses T-cell proliferation
[63][108].
Monoclonal antibodies to the HER2 receptor are used for first-line therapy of patients suffering from HER2/neu-positive subtypes of BC
[64][109] However, about a year after the end of treatment, most patients become immune to the monoclonal antibody drug
[65][110]. It was shown in vitro on cell lines SK-BR-3 and BT-474 that exosomes overexpress HER2 and are able to bind selectively to monoclonal antibodies
[66][111]. Similar results were obtained on exosomes obtained from the blood of treated patients
[65][110]. Probably, exosomes of HER2-positive BC subtypes competitively inhibit monoclonal antibodies, reducing their therapeutic effect. Additionally, resistance to anti-HER2 drugs has been shown to be associated with increased levels of transforming growth factor beta-1 (TGF-β1) and PD-L1
[65][110].