Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ntalane Sello Seroka and Version 3 by Mohammad Raja Ismail AL-SOEIDAT.
The sun is considered a source of clean, renewable energy, and the most abundant. With silicon being the element most used for the direct conversion of solar energy into electrical energy, solar cells are the technology corresponding to the solution of the problem of energy on our planet. Solar cell fabrication has undergone extensive study over the past several decades and improvement from one generation to another.
- solar cells
- semiconductor
- thin films
1. Introduction
Their contributions to sustainability and quality of life have led to the desire to develop, manufacture, and find new technologies based on renewable energy resources. Solar energy has proven to be sustainable and has attracted great attention, with the sun considered the most abundant source of clean, renewable energy. This makes solar cell technology economically viable and sustainable and allows for potential reductions in greenhouse gases, thus making it an ideal source of energy while avoiding shortcomings associated with energy and the environment [1][2][3][4]. In addition[1, solar energy systems are increasingly recognized for their role in achieving global decarbonization targets2, supporting energy independence3, and creating new economic opportunities in both developed and emerging economies4].
Photovoltaics have the ability to generate electrical energy at a lower cost and they are eco-friendly. Thin film solar cells are favorable candidates in the field of photovoltaics because of their minimum material usage and rising efficiencies. The three most commonly known thin film technologies that are extensively studied and still under intensive investigation include α-silicon (α-Si), copper indium gallium selenide (CIGS), and star absorber materials, such as cadmium telluride (CdTe). The conventional inorganic silicon modules, interchangeably known as first generation solar cells, are the leading solar technology for the majority of residential and industrial markets. Presently, crystalline silicon wafers based on a high quality float zone have realized a competitive advantage over traditional solar cells via the utilization of the carrier-selective layer approach [5][8].
The amorphous silicon incorporated into an intrinsic hydrogen layer placed on one or two sides of the float zone silicon wafer serves as a passivation layer for pre-induced carrier-selective contact. Moreover, the aforementioned architecture recorded a power conversion efficiency of 26.6%, derived from the matrix of interdigitated back contact and heterojunction technologies, approaching the theoretical limit power conversion efficiency (PCE) of 29.1% for a silicon solar cell. In spite of its interesting characteristics and strong scalability for industrial and commercial use, the fabrication costs of this technology have halted, thereby making solar panels costly [5][8].
Thus, these drawbacks and limitations have led to the desire to research alternative materials such as (CIGS) and (CdTe), identified as second generation thin film photovoltaics, to adequately compensate for the inability of silicon photovoltaics to provide feasible manufacturing and production and generate inexpensive energy. Although crystalline silicon solar cells currently possess greater than 55% of the market share, the module efficiencies of CIGS and CdTe technologies almost rival that of crystalline solar cells, with recorded efficiencies of 21.4% and 21.6%, respectively [5][6][7][8][8,9,10,11].
Parallel with the emerging materials utilized in thin film technology, most focus is on perovskite solar technology and organic solar cells, as well as dye-sensitized solar cells (DSSCs). These plastic single-junction-based devices exhibit efficiencies of 25.5% [9][12], 16–18.1% [9][10][11][12][13][12,13,14,15,16] and 13% [9][14][12,17], respectively. These are third generation solar cells also known as plastic solar cells. Similarly, exfoliated tungsten telluride (WTe) flake (CiGSe) based multilayer thin films have been reported with a PCE of 10.87%. Furthermore, the structural manipulations of the active materials for these cells have garnered immense momentum due to their ease of production, flexibility, and simple fabrication methodologies [14][15][17,18].
Several studies have reported on these low-cost, complexity-free, and easily manufactured organic solar cells, more especially the π–conjugated polymers. The comprehensive scientific contribution towards the area of application-specific properties of π–conjugated polymers (viz., design, modelling, and fabrication) in the quest to match the electrical conducting properties exhibited by chemically doped polyacetylene is noteworthy. The aforementioned optoelectronic properties have opened technological interest in various applications, such as nano-electronics, sensors, internet of things (IOT), energy storage photodetectors, memory devices, field effect transistors (FETs), nonlinear optical devices, electrochromic, light emitting diodes (LEDs), and photovoltaics [15][16][17][18][19][20][18,19,20,21,22,23].
The conjugated polymers have interesting features owing to the side-chains present in the polymeric materials, which result in significant thermal stability and solubility, and ultimately promote the fabrication of these nanostructure-based materials by deposition with simple techniques, such as spin coating—hence, their inexpensive and simple solution processability. Their single- and double-bond characteristics and their interesting and adequate π-excessive nature have opened a window of opportunity for newly classified advanced materials in the arena of photonics and electronics [20][21][23,24].
TFor the following contentsis study, we propose to utilize novel polycrystalline silicon semiconductor thin film raw material extracted from sugarcane bagasse ash (SCBA) for solar applications. Currently SCBA is being used as the source of fuel in power-generation boilers to produce electricity. Recent studies have shown that SCBA is rich with mineral content including silicon, iron, and other minerals. The silicon is found mainly in the form of silicic acid and constitutes about 380 kg/ha of silicon composition. It is worth noting that pure silicon is further obtained from silica (SiO2) and it has been reported that the silica is biocompatible and eco-friendly [22][23][25,26].
The disposal of bagasse ash waste, therefore, poses a dumping challenge to the environment. Currently, there is very little published information available on the production of silicon nanoparticles from sugarcane bagasse ash for solar applications. Furthermore, quartz is at present the main source of nano silicon. It is obtained by sand mining, which has detrimental effects on the environment such as land degradation, erosion, fissures, and adverse effects on water supply and quality. Metallurgical-grade silicon (MG-Si) (600,000 tons/year) is produced through high-temperature carbothermal reduction of quartz, which requires an energy input of 50 kWh/kg [23][24][26,27].
2. Overview of Solar Cell
2.1. Solar Cell Principle of Operation
Solar cells are mainly described based on their architecture; some consist mostly of metals (inorganic thin films), some nanomaterials (QD), some polymers (referred to as organic), etc. Traditionally, solar cells are electronic devices focused on converting sunlight into direct electrical energy as a consequence of the photoelectric effect from metals and inorganic semiconductors [25][28]. Figure 12 below shows a typical p-n junction silicon solar cell. The generation of electricity comes from the photons (light particles) when sunlight energy shines on the cell. Electrons (−) are ejected from doped Si (n-type) and move across to the positive (+) doped Si (p-type) material as illustrated in Figure 12 below.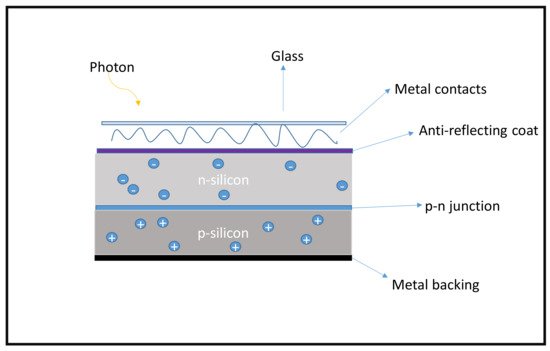
2.1.1. Electronic Structure and Doping Mechanisms in Crystalline Silicon
Electronic Structure
For the originality of electrical conductivity and advanced optical properties, namely low band-gaps in inorganics, it is critical to assimilate the reactivity of these materials, whose electronic configuration is exhibited by doping agents in comparison with undoped inorganic materials. It is well known that the electronic structure of silicon is [Ne] 3s2 3p2, whereby valence electrons avail themselves to form four-bonds with neighboring (adjacent) atoms [26][29].Doping Mechanisms of Silicon Materials
The tailoring of materials is performed to attain electrical properties and semiconductor characteristics via doping. This process involves the introduction of foreign materials and/impurities into the bare material, known as the intrinsic semiconductor, to successfully modulate its optical, electrical, and structural properties, ultimately labelled as the extrinsic semiconductor material. As a result of doping, chemical changes are induced within the silicon material, resulting in the generation of charges which migrate within the silicon matrix. There are various ways and routes to carry out doping including redox, in situ, chemical (gaseous and solution), induced-radiation doping, and charge injection. Additionally, these can be further distinguished by the type of electron transfer, such as doping via reducing agent (n-type) or oxidizing agent (p-type) [26][27][28][29,30,31]. 1. P-type Doping-
P-type Doping
2. N-type Doping
- 2.
-
N-type Doping
2.2. Important Parameters in a Solar Device
Principle of Charge Separation within a Solar Device
The concept of heterojunction cells relies on the efficient dissociation of an exciton, and the donor and acceptor material’s proximity factor plays a crucial role. The material thickness facilitates the optimum exciton diffusion length, usually a few tenths of a nanometer. Typically, the thickness of the active layer for organic semiconductors is in the range 80–200 nm. Recently, bulk heterojunctions have been deposited via co-sublimation of small molecules and/or the spin coating technique, using mixtures of polymers [30][33]. The shortcomings faced in heterojunction structures derive from the fact that hole and electron transportation to the electrodes is required in order to promote the separation of charge carriers in order to reach their corresponding electrodes (cathode and anode). For instance, if the individual layer and a given bilayer structure are larger than that of the exciton diffusion length, it is most likely the excitons will recombine as shown in Figure 3, and result in the loss photon indicated by the exciton with the star. Although if the excitons generated are in close proximity to the interface, there is likelihood that they can be separated into free charge carriers and thus diffuse or drift towards their corresponding electrodes, as shown in Figure 23 [30][31][32][33,34,35].It is imperative to know the solar cell performance, as shown in Figure 34, which can be carried out by determining various factors including the fill factor, efficiency, short-circuit current density, and open-circuit voltage [33][36]. As a result of irradiation, the open-circuit voltage (VOC) is cross-examined as the difference in the cell’s potential at the terminals when there is zero current flow through the terminals. Interestingly, the short-circuit current density (Jsc) is produced from the cell upon irradiation at zero potential.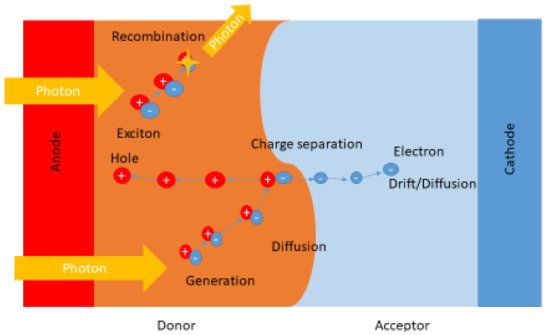 In addition, the fill factor (FF) is known to be the ratio between the cell’s maximum power, shown in Equation (1) where Vmpp and Jmpp denote voltage at the maximum power point and current at the maximum power point, respectively.
In addition, the fill factor (FF) is known to be the ratio between the cell’s maximum power, shown in Equation (1) where Vmpp and Jmpp denote voltage at the maximum power point and current at the maximum power point, respectively.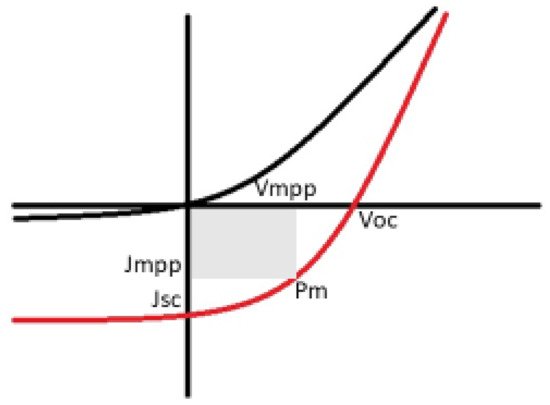 Ultimately the power conversion efficiency (PCE) is determined to be the ratio of the cell’s overall output power to incident radiant power.Innovative designs have made radical developments over the years, whereby thin film solar cell technology results in alternate material device structures. The rising efficiencies of thin film solar cells, in particular perovskite with 23% of the market share, have received significant attention in the photovoltaic market, mostly in the integrated photovoltaic (BIV) field. Plastic solar cells (perovskite) have been rising in efficiency for over two decades, giving them potential as thin film PVs [36][39]. Thin film solar cells are favorable candidates in the field of photovoltaics because of their minimum material usage and rising efficiencies. The three most commonly known thin film technologies extensively studied and still under intensive investigations include α-silicon (α-Si), copper indium gallium selenide (CIGS), and star absorber materials such as cadmium telluride (CdTe). Photovoltaics have the ability to generate electrical energy at a lower cost and they are environmentally friendly. In spite of the aforementioned property (the power conversion from these devices with an enormous amount of the sun’s energy), these photovoltaic devices still produce about 30% or less of electricity from approximately 80–90% of the sun’s energy absorbed [36][37][39,40].
Ultimately the power conversion efficiency (PCE) is determined to be the ratio of the cell’s overall output power to incident radiant power.Innovative designs have made radical developments over the years, whereby thin film solar cell technology results in alternate material device structures. The rising efficiencies of thin film solar cells, in particular perovskite with 23% of the market share, have received significant attention in the photovoltaic market, mostly in the integrated photovoltaic (BIV) field. Plastic solar cells (perovskite) have been rising in efficiency for over two decades, giving them potential as thin film PVs [36][39]. Thin film solar cells are favorable candidates in the field of photovoltaics because of their minimum material usage and rising efficiencies. The three most commonly known thin film technologies extensively studied and still under intensive investigations include α-silicon (α-Si), copper indium gallium selenide (CIGS), and star absorber materials such as cadmium telluride (CdTe). Photovoltaics have the ability to generate electrical energy at a lower cost and they are environmentally friendly. In spite of the aforementioned property (the power conversion from these devices with an enormous amount of the sun’s energy), these photovoltaic devices still produce about 30% or less of electricity from approximately 80–90% of the sun’s energy absorbed [36][37][39,40].
