Metastatic castration-resistant prostate cancer (mCRPC) is a challenging disease to treat, with poor outcomes for patients. One antitumor vaccine, sipuleucel-T, has been approved as a treatment for mCRPC. DNA vaccines are another form of immunotherapy under investigation. DNA immunizations elicit antigen-specific T cells that cause tumor cell lysis, which should translate to meaningful clinical responses. They are easily amenable to design alterations, scalable for large-scale manufacturing, and thermo-stable for easy transport and distribution. Hence, they offer advantages over other vaccine formulations. However, clinical trials with DNA vaccines as a monotherapy have shown only modest clinical effects against tumors. Standard therapies for CRPC including androgen-targeted therapies, radiation therapy and chemotherapy all have immunomodulatory effects, which combined with immunotherapies such as DNA vaccines, could potentially improve treatment. In addition, many investigational drugs are being developed which can augment antitumor immunity, and together with DNA vaccines can further enhance antitumor responses in preclinical models.
- DNA vaccine
- combination therapy
- prostate cancer
- androgen deprivation
- chemotherapy
- radiation
- immune checkpoint blockade
- TLR agonist
- IDO inhibitor
1. Introduction
Prostate cancer has been generally viewed as an immunologically “cold” tumor (i.e., devoid of infiltrating lymphocytes), because trials evaluating T-cell checkpoint blockade therapies, including anti-PD-1 or anti-CTLA-4, have shown little benefit for all but a small number of patients with prostate cancer [1][2][3][4]. This lack of response has been partly attributed to lower numbers of tumor-infiltrating CD8+ T cells in prostate tumors relative to many other solid tumor types that benefit from checkpoint blockade therapy [5]. Prostate cancers also have lower mutational burdens, suggesting a lower number of potential tumor-specific mutation-associated neoantigens for CD8+ T cells to recognize [6]. Although the presence of tumor infiltrating CD8+ T cells has been associated with favorable long-term outcomes for most tumor types, this association has been controversial in prostate cancer. In fact, several reports have associated a higher frequency of CD8+ prostate-infiltrating lymphocytes with shorter time to disease progression [7][8]. Emerging research also suggests that the prostate tumor microenvironment contains elevated levels of myeloid-derived suppressor cells (MDSCs) and indoleamine 2,3 dioxygenase (IDO), contributing to an immunosuppressive environment [9][10]. Together, these findings have suggested that the microenvironments of prostate tumors may be different from those of other solid tumors and that additional immune regulatory cells, cytokines, or other metabolic factors such as hypoxia may affect the function of tumor-infiltrating CD8+ T cells and the response of prostate tumors to immune therapies [11].
Despite the poor response of prostate tumors to T-cell checkpoint blockade therapies, prostate cancer clearly can respond to immune-targeted therapies. Vaccines, agents able to activate and expand tumor-associated T cells, have demonstrated clinical activity in prostate cancer [12][13][14][15][16]. In fact, only one therapeutic cancer vaccine has been FDA-approved for human use, sipuleucel-T (Sip-T), which is an autologous cellular cancer vaccine for patients with mCRPC [17]. Sip-T was approved on the basis of randomized clinical trials demonstrating improved overall survival of treated patients [18][19][20]. In contrast, many antitumor vaccines investigated for patients with melanoma, a disease characterized by many more tumor-infiltrating CD8+ lymphocytes, have failed to demonstrate significant clinical benefit to mCRPC patients. This suggests that vaccines may skew the population of CD8+ T cells, or in some cases, permit infiltration of tumors by CD8+ T cells, an approach that might work best in tumors with few tumor-infiltrating lymphocytes. Patients with newly diagnosed prostate cancer who were treated with Sip-T prior to prostatectomy had significantly more tumor-infiltrating T cells [21]. This suggests that agents, such as T-cell checkpoint molecules that can modulate the function of these tumor-infiltrating lymphocytes, might best be combined with antitumor vaccines. This approach was tested in a murine model of prostate cancer, in which TRAMP mice were treated with the cellular vaccine GVAX (a cellular vaccine made from gene-modified tumor cells), anti-CTLA-4 blocking antibody, or the combination. Decreased tumor grade and increased lymphocytic infiltration of tumors was observed only with the combination treatment [22]. This combination was then evaluated in a small clinical trial in which patients with advanced prostate cancer were treated with the GVAX vaccine in combination with ipilimumab [23]. Specifically, 20% of patients experienced 50% or greater declines in serum PSA levels, suggesting clinical activity. Unfortunately, GVAX alone was not further pursued after early failures in two phase 3 trials [24][25]. In one study, patients were treated with GVAX or docetaxel and prednisone, however, it was terminated early due to a data monitoring committee determination that the study had less than 30% chance of meeting its primary endpoint of increased overall survival [24]. In another study, GVAX combined with docetaxel was compared to docetaxel with prednisone in patients with CRPC, where it was determined that overall survival was significantly shorter in the GVAX arm [25]. Due to the decision to not further pursue GVAX as a treatment, the combination approach of GVAX with ipilimumab was not further explored. Notwithstanding, this demonstrates that combination approaches, using antitumor vaccines with agents able to modulate the infiltration or activity of immune effector cells elicited with vaccination, are reasonable for further evaluation in clinical trials.
Although Sip-T has shown a significant but arguably modest effect in the treatment of mCRPC, and could theoretically be combined with other agents, the cost associated with this treatment makes it less accessible to the majority of patients and makes combination approaches potentially prohibitively costly [26]. DNA vaccines are significantly cheaper, can be used off-the-shelf, and several have already been approved for use in veterinary medicine [27]. Many therapies that are currently in use and in development also have immunomodulatory effects, which can be leveraged to further enhance the antitumor efficacy of DNA vaccines. Combining DNA vaccines with other therapies is consequently a valid approach for the treatment of mCRPC, and these combinations will be the focus of this review.
2. DNA Vaccines as Treatments for Prostate Cancer
Cancer vaccines act by inducing a specific, and ideally long-lasting, immune response against tumor antigens. Various types of cancer vaccines have been tested, with varied mechanisms to elicit an immune response to either arrest cancer progression or prevent tumor recurrence. In the case of prostate cancer, these have included cell-based vaccines, such as dendritic cell vaccines (e.g., Sip-T) or whole tumor cells (e.g., GVAX), protein/peptide vaccines, viral/bacterial-based vaccines (e.g., PROSTVAC, Bavarian Nordic Immunotherapies), and gene-based vaccines, including RNA and DNA vaccines [28]. Vaccines can target antigens shared by tumors from different individuals, or alternatively, neoantigen vaccines are also being explored, which are specific to mutations arising in individual tumors. Theoretically, mutation-associated neoantigen vaccines should be less susceptible to pre-existing T cell tolerance, because the antigens targeted should not be presented in the thymus [28]. However, the small number of tumor-specific mutations in prostate cancer may ultimately limit the feasibility of neoantigen vaccines as an approach for prostate cancer. Although several vaccine approaches have been evaluated in clinical trials for patients with prostate cancer, and reviewed elsewhere, our focus here will be on DNA vaccines.
DNA vaccines are simple vehicles for in vivo transfection and antigen production, consisting of a circular piece of DNA that encodes the antigen of interest under the control of a eukaryotic promoter [29]. DNA vaccines have several advantages over other vaccine platforms. They are easily amenable to design alterations, scalable for large-scale manufacturing, not infectious, not restricted to individuals of a defined MHC type, and thermostable for easy transport and distribution. DNA vaccines are administered by one of several delivery methods such as gene gun to the epidermis, intramuscular injection [30], or intradermal injection. Conceptually, transfected dendritic cells, or dendritic cells cross-presenting antigen produced by bystander transfected cells, travel from the site of delivery to draining lymph nodes, where antigen presentation and T-cell activation occur [31]. Intramuscular injection results in transfection of myocytes that, lacking costimulatory molecules and MHC II molecules, transfer antigen to professional antigen-presenting cells, which can cross-present the antigen to CD8+ T cells [32]. The route of immunization can affect the resulting type of immune response [33].
DNA vaccines are currently not approved for use in humans but have been approved for the treatment of West Nile virus in horses [34] and canine melanoma [35]. However, human DNA vaccines are an active field of research, with clinical trials ongoing in a variety of cancers and infectious disease [36][37][38][39][40][41][42][43][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77].
Identifying target antigens is critical in vaccine design. As described above, while tumor-specific mutation-associated neoantigens are being explored, most prior evaluation of prostate cancer vaccines has focused on antigens specific to prostate cancer and shared by multiple individuals. Ideally, the target should be only expressed in the prostate (or more specifically, the tumor) or at the least highly expressed in the prostate tumor compared to regular tissues to minimize off-tumor cytotoxicity. At least four proteins have been identified as potential immune targets for DNA vaccines that are currently or have been explored in clinical trials for patients with prostate cancer: prostatic acid phosphatase (PAP), prostate-specific antigen (PSA), prostate-specific membrane antigen (PSMA), and the androgen receptor (AR) [44][45]. Preclinical studies investigating the use of DNA vaccines for prostate cancer have recently been reviewed by our group and others [46]. A summary of previous and ongoing clinical trials conducted with DNA vaccines in patients with prostate cancer is shown in Table 1.
Table 1. Clinical trials with DNA vaccines (both as monotherapy and in combination) in prostate cancer to date.
|
Vaccine antigen |
Vaccine Name |
Combination |
Phase (Number Treated) |
Rationale/Approach |
Major Finding |
NCT trial number |
Ref. |
|
Rhesus PSA |
pVAXrcPSAv531 |
– |
1 (N = 15) |
Dose escalation. Safety, changes in PSA kinetics, and detection of PSA-specific immune responses in patients with nmCSPC |
Vx was safe. No changes in PSA kinetics. 14/15 patients had PSA-specific immune responses due to vx or ADT |
NCT00859729
|
[47] |
|
PSA |
pVAX/PSA |
– |
1 (N = 8) |
Dose escalation. Safety and detection of PSA-specific cellular immunity in CRPC |
Vx was safe. At highest dose (900 μg), PSA-specific cellular and humoral immunity detected |
- |
[15] |
|
PSA + PSMA |
INO-5150 |
+ IL-12 DNA plasmid (INO-9012)
|
1 (N = 62) |
Safety, tolerability, immune response to PSA and PSMA, PSA doubling time and PSA kinetics. Patients with biochemically recurrent PCa (nmCSPC) |
Vx was safe, and 53/62 patients were progression-free after 72 weeks. PSA doubling time increased in patients with pretreatment PSA doubling time <12 months, and 47/62 patients had PSA- or PSMA-specific immunity |
NCT02514213 |
[48] |
|
PSMA + PRAME |
MKC1106-PP |
– |
1 (N = 24) |
Fixed DNA plasmid (prime) and two different doses of peptide boost (low/high). Safety, PSA or PRAME specific immune response, clinical benefit (stable disease) in CRPC |
Vx was safe, and 4/10 showed PSA decline or stable disease for >6 months. Association between antigen-specific T cells above baseline and disease control (stable disease >6 months) |
NCT00423254 |
[45] |
|
NY-ESO1 |
pPJV7611 |
– |
1 (N = 16) |
Safety and immune response in patients with different malignancies, including 9 with metastatic prostate cancer |
Vx was safe. All 10 patients had CD4+ immune responses, and 2/10 patients had CD8+ immune responses |
NCT00199849 |
[49] |
|
AR LBD
|
pTVG-AR (MVI-118) |
– |
1 (N = 40) |
Safety, immune response, median time to PSA progression, and 18-month PSA progression free survival in patients with mCSPC |
Vx was safe, and 14/30 evaluated patients developed AR-specific cellular immunity. Patients with T cell immunity had significantly longer time to PSA progression |
NCT02411786
|
[44] |
|
PAP |
pTVG-HP (MVI-816) |
– |
1 (N = 22) |
Dose escalation. Safety, PAP-specific immune response, PSA doubling time in patients with nmCSPC |
Vx was safe, and 9/22 patients developed PAP-specific CD4+ and/or CD8+ cell proliferation. PSA doubling time increased from 6.5 months pretreatment to 8.5 months post-treatment and 9.3 months to 1-year post-treatment |
NCT00582140
|
[50] |
|
PAP |
pTVG-HP (MVI-816) |
– |
1/2 (N = 16) |
Tested two schedules: 6 immunizations every 2 weeks, then every 3 months for up to 2 years versus 6 immunizations every 2 weeks, then immunized based on results from immune monitoring. In patients with nmCRPC |
Immune monitoring did not lead to superior schedule. Antigen-specific T cells elicited persisted over time |
NCT00849121
|
[51] |
|
PAP |
pTVG-HP (MVI-816) |
– |
2 (N = 99) |
Randomized to pTVG-HP with GM-CSF versus GM-CSF alone in patients with nmCSPC and PSA doubling time < 12 months |
Two-year metastasis-free survival was not different overall between study arms. Patients with a pretreatment PSA doubling time < 3 months, MFS was significantly longer in vx arm. Decreased NaF uptake by PET/CT imaging suggested vx affected bone micrometastatic disease |
NCT01341652
|
[12] |
|
PAP |
pTVG-HP (MVI-816) |
+ pembrolizumab |
1 / 2 (N = 66) |
Assess pTVG-HP with pembrolizumab (concurrent) or pTVG-HP vx first followed by pembrolizumab (sequential) in patients with mCRPC |
Median time to radiographic progression was not different; 8/13 patients treated concurrently and 1/12 patients treated sequentially had PSA declines from baseline. PSA declines associated with PAP-specific cellular immunity and CD8+ tumor infiltration. Expansion cohorts ongoing |
NCT02499835
|
[42] [52] |
|
PAP |
pTVG-HP (MVI-816) |
+ Sip-T |
2 (N = 18) |
Assessed whether pTVG-HP could augment Sip-T antitumor efficacy in patients with mCRPC |
Treatment was safe, and 11/18 patients developed PAP-specific cellular immunity. Higher antibody immunity observed in patients receiving pTVG-HP boost compared to Sip-T alone. Median time to progression was not significantly different |
NCT01706458
|
[53] |
|
PAP |
pTVG-HP (MVI-816) |
+ nivolumab |
2 (N = 21–41) |
Assess the safety and PSA complete response rate using pTVG-HP with nivolumab in patients with nmCSPC |
Ongoing |
NCT03600350
|
– |
|
PAP and AR LBD |
pTVG-HP (MVI-816) and pTVG-AR (MVI-118) |
+ pembrolizumab |
2 (N = 60) |
Assess efficacy (6m PFS) of one versus two DNA vaccines, with PD-1 blockade in patients with mCRPC |
Ongoing |
NCT04090528
|
– |
|
Mutation-associated neoantigens |
|
+ PROSTVAC + ipilimumab + nivolumab |
1 (N = 20) |
Will elucidate safety and immune response to a shared antigen vaccine and tumor-specific antigen DNA vaccine with ICB |
Ongoing |
NCT03532217
|
– |
Abbreviations used: ICB immune checkpoint blockade, mCRPC metastatic castration-resistant prostate cancer, nmCSPC non-metastatic castration-sensitive prostate cancer, MFS metastasis-free survival, NaF sodium fluoride, PCa prostate cancer, vx vaccination.
The most studied antigen in human prostate cancer vaccine trials is prostatic acid phosphatase (PAP), the same target of the sipuleucel-T vaccine. PAP is expressed at high levels in the epithelium in prostate cancer patients [54]. In a Phase 1/2 dose-escalation study (NCT00582140), patients received pTVG-HP at three different doses: 100, 500, and 1500 μg at 14-day intervals for six doses. The vaccine was well tolerated, and of the 22 patients on study, 6 patients developed at least a threefold increase in PAP-specific CD4+ proliferative T cells, and 3 patients developed at least a threefold increase in PAP-specific CD8+ proliferative T cells [50]. Immune responses were detected at each dose level. Furthermore, 7 of 22 patients experienced an increase in PSA doubling time [50]. In a separate phase 1 trial, two different schedules of pTVG-HP were assessed in 17 patients [51]. The first schedule was fixed, with six 14-day interval immunizations, followed by boosters every 3 months for up to 2 years. The second schedule received 6 immunizations at 14-day intervals, after which they were monitored for PAP-specific immune responses to guide subsequent immunizations. The study identified that multiple DNA immunizations were required to elicit and maintain a long-term PAP-specific immune response. Given these findings, a randomized phase 2 study (NCT01341652) was conducted to determine whether vaccination could delay the development of metastatic disease in patients with biochemically recurrent disease [12]. Ninety-nine patients with castration-sensitive prostate cancer, without radiographic evidence of metastases and a PSA doubling time of less than 12 months, were randomized to receive pTVG-HP and GM-CSF adjuvant or GM-CSF alone. The results did not show an overall difference in 2-year metastasis-free survival between the two cohorts. However, a subset of patients with rapidly progressing disease (as determined by rapid PSA doubling time) and treated with pTVG-HP were identified to have longer metastasis-free survival (MFS) [12]. As an exploratory endpoint, sodium fluoride (18F-NaF) PET/CT was used to identify micrometastatic bone disease in a subset of patients. Decreases in NaF uptake were observed in pTVG-HP-treated individuals, compared with increases in the GM-CSF control group, suggesting that vaccination had detectable effects on micrometastatic bone disease. Overall, however, the conclusion of this study was that, while this vaccine demonstrated some antitumor efficacy in a subset of patients with rapidly progressing disease, it should not be further pursued as a single agent because the study did not meet the primary endpoint of significantly increased 2-year metastasis-free survival in patients treated with pTVG-HP [12]. Thus, it was deemed that DNA vaccines best be evaluated in combination with other vaccines or other immune-activating agents.
PSA is a classical biomarker for prostate cancer and is currently used as a measure of tumor response or progression. Given the increased expression of PSA in the tumor during disease progression, it has been explored as a possible target for DNA vaccines. In a phase 1 dose-escalation study, a plasmid encoding full-length PSA was given to patients with advanced CRPC in monthly cycles for 5 months. The doses given were 100, 300, and 900 μg of DNA, with 90% of the dose given intramuscularly and 10% of the dose given intradermally. Only the highest dose of 900 μg of DNA elicited PSA-specific cellular and humoral antibody responses [15]. No IL-10 was detected, however, other Th2 cytokines such as IL-4 and IL-6 were detected, suggesting that it is important to not only look at Th1 cytokines but also at potentially immunosuppressive cytokines [13]. To further improve immunogenicity of the PSA vaccine, researchers tested a DNA vaccine encoding the Rhesus PSA gene, which was administered with electroporation to increase transfection of the antigen-presenting cells [47]. Fifteen patients were required to start androgen deprivation therapy before vaccination. Patients received doses of plasmid ranging from 50 to 1600 μg. The DNA vaccine was administered intradermally, followed immediately by electroporation. All patients except one had pre-existing PSA-specific T cells, the frequency of which was increased by either androgen deprivation or by vaccination. However, only the two highest doses, 1000 and 1600 μg elicited immune responses [47].
Prostate-specific membrane antigen (PSMA) has also been tested as a target antigen for vaccination. PSMA is highly expressed in the epithelium in prostate adenocarcinoma [55], making it a promising target for vaccination. A DNA vaccine encoding an HLA-A2 binding epitope of the PSMA gene fused to a fragment of the tetanus toxin was tested in a phase 1/2 dose escalation trial [56]. Patients who were HLA-A2+ were recruited to receive vaccine with or without electroporation, whereas a patient population who were HLA-A2− served as a negative control. Vaccinated patients had a significant increase in PSA doubling time compared to unvaccinated patients, suggesting slower disease progression [56]. In patients that received the vaccine, PSMA-specific T cells were significantly increased postvaccination compared to baseline. A more recent study (NCT02514213) examined the effect of INO-5150, a DNA vaccine consisting of plasmids encoding both PSMA and PSA in patients with biochemically recurrent prostate cancer [48]. Treatment was considered safe with no patients experiencing serious treatment-related adverse events. A quarter of patients in the trial exhibited higher immune responses compared to baseline, to either PSMA or PSA, as detected by IFN-γ ELISPOT or by flow cytometric analysis of antigen-specific T cells.
The androgen receptor is expressed in the prostate and is important for prostate development, function and also plays a role in cancer progression, with AR overexpression identified as a major mechanism of resistance in CRPC [57]. A DNA vaccine encoding the ligand-binding domain of the androgen receptor (pTVG-AR) was demonstrated to delay tumor growth in several prostate cancer models [58]. In a phase 1 clinical trial (NCT02411786), pTVG-AR was given to patients with castration-sensitive prostate cancer, on two schedules and with or without the adjuvant GM-CSF. Vaccination was demonstrated to be safe, and one schedule was demonstrated to elicit more AR-specific, interferon-γ (IFN-γ) producing T cells compared to the other schedule [44]. The addition of GM-CSF did not significantly boost AR-specific immunity. Intriguingly, patients who developed cellular immune responses had significantly longer PSA progression-free survival compared to patients who did not develop immune responses. Further evaluation of this vaccine in combination with other agents is anticipated.
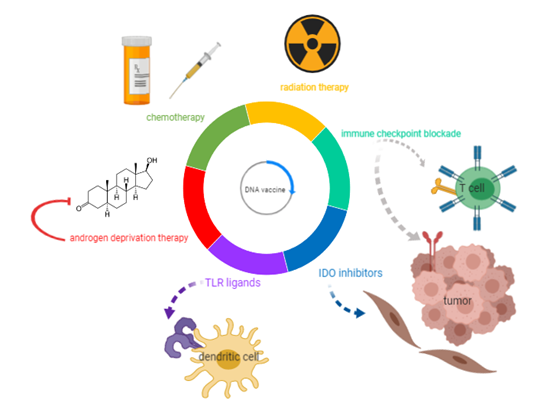
These recent and ongoing studies demonstrate that DNA vaccines are safe, can elicit antigen-specific T-cells, and can have other detectable antitumor effects. However, the direct antitumor effects as measured by changes in serum PSA or objective responses have been underwhelming when these agents have been used as monotherapies. Consequently, these approaches are currently being combined with other immunomodulatory therapies and conventional prostate cancer treatments to determine if there might be synergies that could be exploited to further improve the immunogenicity of DNA vaccines and augment the antitumor response. These approaches are depicted in Figure 1, and the mechanisms of action and evidence supporting their combination with DNA vaccines are summarized in the following sections.
Figure 1. DNA vaccine combinations under evaluation as potential treatments for prostate cancer. With the goal of augmenting the efficacy of DNA vaccines in controlling prostate tumor growth, this review explores DNA vaccine combinations under investigation with current therapies for metastatic castration-resistant prostate cancer (mCRPC) (androgen deprivation, chemotherapy, and radiation), as well as immunomodulatory agents (immune checkpoint blockade, Toll-like receptors (TLR) ligands, and indoleamine 2,3 dioxygenase (IDO) inhibitors).
3. Androgen Deprivation Therapy
Androgen deprivation therapy (ADT) has been a cornerstone therapy for the treatment of recurrent and metastatic prostate cancer. Early studies showed that androgen deprivation, either by surgical or chemical means, causes a decrease in prostate size and induces tumor apoptosis [59]. Androgen deprivation also affects the immune system in various ways. Castration causes an increase in thymus size, whereas testosterone replacement after castration induces thymic regression [60]. Spleens from castrated C57Bl/6 mice have significantly more B cells and significantly fewer CD4+ T cells [61]. Androgen deprivation also causes increased T-cell infiltration into the prostate and prostate tumors. In rodent studies, these infiltrating lymphocytes were initially of Th1 phenotype within the first 30 days after castration, but by 90 days after castration, the infiltrating lymphocytes had a Th17 phenotype [62][63][64][62–64]. In human studies, tumor-infiltrating lymphocytes consist of both effector CD8+ T cells and CD4+ T cells, including regulatory T cells (Treg) [65].
With these different immunomodulatory effects on the immune system, ADT may be logically combined with vaccine-based therapies. In a phase 2 clinical trial assessing the sequencing of Sip-T and androgen deprivation in patients with biochemically recurrent prostate cancer at high risk of metastasis, patients who received Sip-T followed by ADT had increased IFN-γ responses to PA2024 (the PAP/GM-CSF fusion protein vaccine antigen) compared to patients treated with ADT followed by Sip-T at the 6 week time point and increased PA2024-specific T cell proliferation averaged over all time points [66]. In another randomized trial comparing viral PROSTVAC vaccine to the AR antagonist nilutamide, in patients with biochemically recurrent castration resistant prostate cancer, there was no significant difference in time to treatment failure between the vaccine arm versus the nilutamide arm[67] [67]. However, upon PSA progression, patients were offered the combination therapy, adding either PROSTVAC or nilutamide to their treatment regimen. Patients who received PROSTVAC followed by nilutamide had a significantly longer median time to treatment failure of 13.9 months, compared to patients who received nilutamide followed by PROSTVAC with a median time to treatment failure of 5.2 months [67]. The same patients were followed-up 6 years later, and the trend held. Patients that received PROSTVAC first followed by nilutamide had a median overall survival of 6.2 years, significantly longer than patients who received nilutamide first followed by PROSTVAC who had a median overall survival of 3.7 years [68]. A recent study examining GVAX with the T-reg depleting agent cyclophosphamide followed by degarelix, showed that the triple combination resulted in significantly increased time to PSA progression and time to next treatment compared to degarelix alone [69]. Treatment with ADT resulted in CD8+ T cell infiltration into the tumor, however, also increased T-regs and likely immunosuppressive myeloid cells [69].
Androgen deprivation therapy specifically in combination with DNA vaccines has been less explored. Preclinical studies in mouse and rat models using the pTVG-AR DNA vaccine described earlier demonstrated significant antitumor activity when androgen deprivation was combined with vaccination, likely due to increased expression of the androgen receptor within prostate tumors following androgen deprivation [57]. As discussed, this DNA vaccine was evaluated in a phase 1 clinical trial in patients who had recently started ADT. Patients who developed immunity to AR were found to have a prolonged time to castration resistance, consistent with prior rodent studies [44]. Collectively, these data suggest that DNA vaccines should be further explored in combination with androgen deprivation and that the type of androgen deprivation and sequence with vaccination should be further studied.
4. Chemotherapy
Chemotherapy is an overarching term for any cytotoxic drug that targets rapidly dividing cells, including cancer cells. For many years, chemotherapy was the only option for patients with progressive mCRPC. Docetaxel is still considered a first line of treatment for mCRPC, offering a 2–3 months median survival advantage compared to mitoxantrone [70]. A second-generation cytotoxic chemotherapy, cabazitaxel, demonstrated a 2.4-months increase in median survival for patients previously treated with docetaxel, and was approved for patients with mCRPC [71]. Although the survival advantage with chemotherapy is limited for patients with castration-resistant disease, it has been shown to be effective in pain management and improving patient quality of life.
Investigators have long thought that chemotherapy and anticancer vaccines were incompatible due to the immunosuppressive effects of chemotherapy drugs. However, mounting evidence suggests this might not be the case [72]. A preclinical study examining the effects of docetaxel and a poxviral vaccine encoding the self-antigen carcinoembryonic antigen (CEA) in tumor-bearing mice demonstrated that when docetaxel was administered after the vaccine, there was an increased antitumor effect compared to vaccine alone [73]. In a phase 2 clinical trial that assessed whether docetaxel concurrently administered with PROSTVAC could elicit an immune response, there was no observed decrease in T cell responses to PSA in the docetaxel/vaccine group compared to vaccine alone, suggesting chemotherapy did not impair immune responses to vaccine [74]. A randomized multicenter phase 2 trial to evaluate PROSTVAC prior to docetaxel chemotherapy, versus docetaxel chemotherapy alone, was planned to evaluate whether vaccine could improve the overall survival from chemotherapy alone. Unfortunately, this trial was stopped early without meeting its accrual goal [75]. In another small phase 2 clinical trial, high-risk patients with localized disease were treated with neoadjuvant docetaxel and GVAX, followed by radical prostatectomy [76]. Six patients completed the treatment regimen. No serious drug-related adverse events were observed and the median change in prostate-specific antigen (PSA) was minor but decreasing. Of the five patients who completed prostatectomy, four had a down-staging of their Gleason score. Undetectable PSA was achieved in three patients at 2 months after prostatectomy and in two patients at 3 years after prostatectomy, demonstrating that chemoimmuno therapy combinations can result in durable responses possibly due to the disruption of the immunosuppressive microenvironment. Two phase 3 trials examining the combination of docetaxel with GVAX were terminated prematurely due to increased death in the combination arm [77]. Such findings reinforce the need for basic scientific research into the mechanisms of potential interactions and importance of sequence when combining therapies. To our knowledge, the specific combination of chemotherapy with DNA vaccines has not yet been explored in prostate cancer or indeed, in any type of cancer.
References
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, double-blind, phase iii trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 2017, 35, 40–47.
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti–pd-1 antibody in cancer. New Engl. J. Med. 2012, 366, 2443–2454.
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (ca184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712.
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase ii keynote-199 study. J. Clin. Oncol. 2020, 38, 395–405.
- Steele, K.E.; Tan, T.H.; Korn, R.; Dacosta, K.; Brown, C.; Kuziora, M.; Zimmermann, J.; Laffin, B.; Widmaier, M.; Rognoni, L.; et al. Measuring multiple parameters of cd8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J. Immunother. Cancer 2018, 6, 20.
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nat. 2013, 500, 415–421.
- Ness, N.; Andersen, S.; Valkov, A.; Nordby, Y.; Donnem, T.; Al-Saad, S.; Busund, L.T.; Bremnes, R.M.; Richardsen, E., Infiltration of cd8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 2014, 74, 1452–1461.
- Petitprez, F.; Fossati, N.; Vano, Y.; Freschi, M.; Becht, E.; Lucianò, R.; Calderaro, J.; Guédet, T.; Lacroix, L.; Rancoita, P.M.V.; et al. Pd-l1 expression and cd8(+) t-cell infiltrate are associated with clinical progression in patients with node-positive prostate cancer. Eur. Urol. Focus 2019, 5, 192–196.
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M., The tumor immune contexture of prostate cancer. Front. Immunol. 2019, 10, 603.
- Zahm, C.D.; Johnson, L.E.; McNeel, D.G., Increased indoleamine 2,3-dioxygenase activity and expression in prostate cancer following targeted immunotherapy. Cancer Immunol. Immunother. 2019, 68, 1661–1669.
- Prokhnevska, N.; Emerson, D.A.; Kissick, H.T.; Redmond, W.L., Immunological complexity of the prostate cancer microenvironment influences the response to immunotherapy. Adv. Exp. Med. Biol. 2019, 1210, 121–147.
- McNeel, D.G.; Eickhoff, J.C.; Johnson, L.E.; Roth, A.R.; Perk, T.G.; Fong, L.; Antonarakis, E.S.; Wargowski, E.; Jeraj, R.; Liu, G., Phase ii trial of a DNA vaccine encoding prostatic acid phosphatase (ptvg-hp [mvi-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J. Clin. Oncol.2019, 37, 3507–3517.
- Miller, A.M.; Özenci, V.; Kiessling, R.; Pisa, P., Immune monitoring in a phase 1 trial of a psa DNA vaccine in patients with hormone-refractory prostate cancer. J. Immunother. 2005, 28, 389–395.
- Mincheff, M.; Tchakarov, S.; Zoubak, S.; Loukinov, D.; Botev, C.; Altankova, I.; Georgiev, G.; Petrov, S.; Meryman, H.T., Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: A phase i/ii clinical trial. Eur. Urol. 2000, 38, 208–217.
- Pavlenko, M.; Roos, A.K.; Lundqvist, A.; Palmborg, A.; Miller, A.M.; Ozenci, V.; Bergman, B.; Egevad, L.; Hellström, M.; Kiessling, R.; et al. A phase i trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br. J. Cancer 2004, 91, 688–694.
- Zlotocha, S.; Staab, M.J.; Horvath, D.; Straus, J.; Dobratz, J.; Oliver, K.; Wasielewski, S.; Alberti, D.; Liu, G.; Wilding, G.; et al. A phase i study of a DNA vaccine targeting prostatic acid phosphatase in patients with stage d0 prostate cancer. Clin. Genitourin. Cancer 2005, 4, 215–218.
- Handy, C.E.; Antonarakis, E.S., Sipuleucel-t for the treatment of prostate cancer: Novel insights and future directions. Future Oncol. 2018, 14, 907–917.
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-t immunotherapy for castration-resistant prostate cancer. New Engl. J. Med. 2010, 363, 411–422.
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M., Placebo-controlled phase iii trial of immunologic therapy with sipuleucel-t (apc8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol.2006, 24, 3089–3094.
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W., Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-t in advanced prostate cancer. Cancer 2009, 115, 3670–3679.
- Fong, L.; Carroll, P.; Weinberg, V.; Chan, S.; Lewis, J.; Corman, J.; Amling, C.L.; Stephenson, R.A.; Simko, J.; Sheikh, N.A.; et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-t for localized prostate cancer. J. Natl. Cancer Inst. 2014, 106.
- Hurwitz, A.A.; Foster, B.A.; Kwon, E.D.; Truong, T.; Choi, E.M.; Greenberg, N.M.; Burg, M.B.; Allison, J.P., Combination immunotherapy of primary prostate cancer in a transgenic mouse model using ctla-4 blockade. Cancer Res. 2000, 60, 2444–2448.
- van den Eertwegh, A.J.; Versluis, J.; van den Berg, H.P.; Santegoets, S.J.; van Moorselaar, R.J.; van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I.; et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 509–517.
- Higano, C., A phase iii trial of gvax immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (crpc). Proceedings of the 2009 Genitourinary Cancer Symposium, American Society of Clinical Oncology (ASCO), Orlando, FL, USA. 26-28, Feb 2009.
- Small, E., A phase iii trial of gvax immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (crpc). Proceedings of the 2009 Genitourinary Cancer Symposium, American Society of Clinical Oncology (ASCO), Orlando, FL, USA. 26-28 Feb 2009.
- Caram, M.E.V.; Ross, R.; Lin, P.; Mukherjee, B., Factors associated with use of sipuleucel-t to treat patients with advanced prostate cancer. JAMA Netw. Open 2019, 2, e192589.
- Zahm, C.D.; Colluru, V.T.; McNeel, D.G., DNA vaccines for prostate cancer. Pharmacol. Ther. 2017, 174, 27–42.
- Lopes, A.; Vandermeulen, G.; Préat, V., Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146.
- Liu, M.A., DNA vaccines: An historical perspective and view to the future. Immunol Rev. 2011, 239, 62–84.
- Fioretti, D.; Iurescia, S.; Fazio, V.M.; Rinaldi, M., DNA vaccines: Developing new strategies against cancer. J. Biomed. Biotechnol. 2010, 2010, 174378.
- Porgador, A.; Irvine, K.R.; Iwasaki, A.; Barber, B.H.; Restifo, N.P.; Germain, R.N., Predominant role for directly transfected dendritic cells in antigen presentation to cd8+ t cells after gene gun immunization. J. Exp. Med. 1998, 188, 1075–1082.
- Fu, T.M.; Ulmer, J.B.; Caulfield, M.J.; Deck, R.R.; Friedman, A.; Wang, S.; Liu, X.; Donnelly, J.J.; Liu, M.A., Priming of cytotoxic t lymphocytes by DNA vaccines: Requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol. Med. 1997, 3, 362–371.
- Yokoyama, M.; Hassett, D.E.; Zhang, J.; Lindsay Whitton, J., DNA immunization can stimulate florid local inflammation, and the antiviral immunity induced varies depending on injection site. Vaccine 1997, 15, 553–560.
- Dauphin, G.; Zientara, S., West nile virus: Recent trends in diagnosis and vaccine development. Vaccine 2007, 25, 5563–5576.
- Atherton, M.J.; Morris, J.S.; McDermott, M.R.; Lichty, B.D., Cancer immunology and canine malignant melanoma: A comparative review. Vet. Immunol Immunopathol. 2016, 169, 15–26.
- Carter, C.; Houser, K.V.; Yamshchikov, G.V.; Bellamy, A.R.; May, J.; Enama, M.E.; Sarwar, U.; Larkin, B.; Bailer, R.T.; Koup, R.; et al. Safety and immunogenicity of investigational seasonal influenza hemagglutinin DNA vaccine followed by trivalent inactivated vaccine administered intradermally or intramuscularly in healthy adults: An open-label randomized phase 1 clinical trial. PLoS ONE 2019, 14, e0222178.
- Elizaga, M.L.; Li, S.S.; Kochar, N.K.; Wilson, G.J.; Allen, M.A.; Tieu, H.V.N.; Frank, I.; Sobieszczyk, M.E.; Cohen, K.W.; Sanchez, B.; et al. Safety and tolerability of hiv-1 multiantigen pdna vaccine given with il-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus hiv gag vaccine in healthy volunteers in a randomized, controlled clinical trial. PLoS ONE 2018, 13, e0202753.
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562.
- Hannaman, D.; Dupuy, L.C.; Ellefsen, B.; Schmaljohn, C.S., A phase 1 clinical trial of a DNA vaccine for venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine 2016, 34, 3607–3612.
- Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D., Jr.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent hpv vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723.
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A sars DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a phase i clinical trial. Vaccine 2008, 26, 6338–6343.
- McNeel, D.G.; Eickhoff, J.C.; Wargowski, E.; Zahm, C.; Staab, M.J.; Straus, J.; Liu, G., Concurrent, but not sequential, pd-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2018, 9, 25586–25596.
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of vgx-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 e6 and e7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088.
- Kyriakopoulos, C.E.; Eickhoff, J.; Ferrari, A.C.; Schweizer, M.T.; Wargowski, E.; Olson, B.M.; McNeel, D.G., Multicenter phase 1 trial of a DNA vaccine encoding the androgen receptor ligand binding domain (ptvg-ar, mvi-118) in patients with metastatic prostate cancer. Clin. Cancer Res. 2020.
- Weber, J.S.; Vogelzang, N.J.; Ernstoff, M.S.; Goodman, O.B.; Cranmer, L.D.; Marshall, J.L.; Miles, S.; Rosario, D.; Diamond, D.C.; Qiu, Z.; et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J. Immunother. 2011, 34, 556–567.
- McNeel, D.G.; Becker, J.T.; Johnson, L.E.; Olson, B.M., DNA vaccines for prostate cancer. Curr. Cancer Ther. Rev. 2012, 8, 254–263.
- Eriksson, F.; Tötterman, T.; Maltais, A.-K.; Pisa, P.; Yachnin, J., DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine 2013, 31, 3843–3848.
- Shore, N.D.; Morrow, M.P.; McMullan, T.; Kraynyak, K.A.; Sylvester, A.; Bhatt, K.; Cheung, J.; Boyer, J.D.; Liu, L.; Sacchetta, B.; et al. Cd8(+) t cells impact rising psa in biochemically relapsed cancer patients using immunotherapy targeting tumor-associated antigens. Mol. Ther. 2020, 28, 1238–1250.
- Gnjatic, S.; Altorki, N.K.; Tang, D.N.; Tu, S.M.; Kundra, V.; Ritter, G.; Old, L.J.; Logothetis, C.J.; Sharma, P., Ny-eso-1 DNA vaccine induces t-cell responses that are suppressed by regulatory t cells. Clin. Cancer Res. 2009, 15, 2130–2139.
- McNeel, D.G.; Dunphy, E.J.; Davies, J.G.; Frye, T.P.; Johnson, L.E.; Staab, M.J.; Horvath, D.L.; Straus, J.; Alberti, D.; Marnocha, R.; et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage d0 prostate cancer. J. Clin. Oncol.2009, 27, 4047–4054.
- McNeel, D.G.; Becker, J.T.; Eickhoff, J.C.; Johnson, L.E.; Bradley, E.; Pohlkamp, I.; Staab, M.J.; Liu, G.; Wilding, G.; Olson, B.M., Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3692–3704.
- Scarpelli, M.; Zahm, C.; Perlman, S.; McNeel, D.G.; Jeraj, R.; Liu, G., Flt pet/ct imaging of metastatic prostate cancer patients treated with ptvg-hp DNA vaccine and pembrolizumab. J. Immunother. Cancer 2019, 7, 23.
- Wargowski, E.; Johnson, L.E.; Eickhoff, J.C.; Delmastro, L.; Staab, M.J.; Liu, G.; McNeel, D.G., Prime-boost vaccination targeting prostatic acid phosphatase (pap) in patients with metastatic castration-resistant prostate cancer (mcrpc) using sipuleucel-t and a DNA vaccine. J. Immunother. Cancer 2018, 6, 21–21.
- Graddis, T.J.; McMahan, C.J.; Tamman, J.; Page, K.J.; Trager, J.B., Prostatic acid phosphatase expression in human tissues. Int. J. Clin. Exp. Pathol. 2011, 4, 295–306.
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G., Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998, 52, 637–640.
- Chudley, L.; McCann, K.; Mander, A.; Tjelle, T.; Campos-Perez, J.; Godeseth, R.; Creak, A.; Dobbyn, J.; Johnson, B.; Bass, P.; et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency cd8(+) t-cell responses and increases psa doubling time. Cancer Immunol. Immunother. 2012, 61, 2161–2170.
- Olson, B.M.; Gamat, M.; Seliski, J.; Sawicki, T.; Jeffery, J.; Ellis, L.; Drake, C.G.; Weichert, J.; McNeel, D.G., Prostate cancer cells express more androgen receptor (ar) following androgen deprivation, improving recognition by ar-specific t cells. Cancer Immunol. Res. 2017, 5, 1074–1085.
- Johnson, L.E.; Olson, B.M.; McNeel, D.G., Pretreatment antigen-specific immunity and regulation - association with subsequent immune response to anti-tumor DNA vaccination. J. Immunother. Cancer 2017, 5, 56.
- Huggins, C.; Stevens, R.; Hodges, C.V., Studies on prostatic cancer: Ii. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg.1941, 43, 209–223.
- Olsen, N.J.; Watson, M.B.; Henderson, G.S.; Kovacs, W.J., Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinol. 1991, 129, 2471–2476.
- Viselli, S.M.; Stanziale, S.; Shults, K.; Kovacs, W.J.; Olsen, N.J., Castration alters peripheral immune function in normal male mice. Immunol. 1995, 84, 337–342.
- Roden, A.C.; Moser, M.T.; Tri, S.D.; Mercader, M.; Kuntz, S.M.; Dong, H.; Hurwitz, A.A.; McKean, D.J.; Celis, E.; Leibovich, B.C.; et al. Augmentation of t cell levels and responses induced by androgen deprivation. J. Immunol. 2004, 173, 6098–6108.
- Mercader, M.; Bodner, B.K.; Moser, M.T.; Kwon, P.S.; Park, E.S.; Manecke, R.G.; Ellis, T.M.; Wojcik, E.M.; Yang, D.; Flanigan, R.C.; et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14565–14570.
- Morse, M.D.; McNeel, D.G., T cells localized to the androgen-deprived prostate are th1 and th17 biased. Prostate 2012, 72, 1239–1247.
- Sorrentino, C.; Musiani, P.; Pompa, P.; Cipollone, G.; Di Carlo, E., Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory t lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin. Cancer Res. 2011, 17, 1571–1581.
- Antonarakis, E.S.; Kibel, A.S.; Yu, E.Y.; Karsh, L.I.; Elfiky, A.; Shore, N.D.; Vogelzang, N.J.; Corman, J.M.; Millard, F.E.; Maher, J.C.; et al. Sequencing of sipuleucel-t and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: A phase ii randomized trial. Clin. Cancer Res. 2017, 23, 2451–2459.
- Arlen, P.M.; Gulley, J.L.; Todd, N.; Lieberman, R.; Steinberg, S.M.; Morin, S.; Bastian, A.; Marte, J.; Tsang, K.Y.; Beetham, P.; et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J. Urol. 2005, 174, 539–546.
- Madan, R.A.; Gulley, J.L.; Schlom, J.; Steinberg, S.M.; Liewehr, D.J.; Dahut, W.L.; Arlen, P.M., Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin. Cancer Res. 2008, 14, 4526–4531.
- Obradovic, A.Z.; Dallos, M.; Zahurak, M.L.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E.; Allaf, M.E.; Nirschl, T.R.; Chapman, C.G.; O'Neal, T.; et al. T-cell infiltration and adaptive treg resistance in response to androgen deprivation with or without vaccination in localized prostate cancer. Clin. Cancer Res. 2020.
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512.
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154.
- Madan, R.A.; Arlen, P.M., Recent advances revolutionize treatment of metastatic prostate cancer. Future Oncol. 2013, 9, 1133–1144.
- Garnett, C.T.; Schlom, J.; Hodge, J.W., Combination of docetaxel and recombinant vaccine enhances t-cell responses and antitumor activity: Effects of docetaxel on immune enhancement. Clin. Cancer Res. 2008, 14, 3536–3544.
- Arlen, P.M.; Gulley, J.L.; Parker, C.; Skarupa, L.; Pazdur, M.; Panicali, D.; Beetham, P.; Tsang, K.Y.; Grosenbach, D.W.; Feldman, J.; et al. A randomized phase ii study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1260–1269.
- McNeel, D.G.; Chen, Y.H.; Gulley, J.L.; Dwyer, A.J.; Madan, R.A.; Carducci, M.A.; DiPaola, R.S., Randomized phase ii trial of docetaxel with or without psa-tricom vaccine in patients with castrate-resistant metastatic prostate cancer: A trial of the ecog-acrin cancer research group (e1809). Hum. Vaccines Immunother. 2015, 11, 2469–2474.
- Vuky, J.; Corman, J.M.; Porter, C.; Olgac, S.; Auerbach, E.; Dahl, K., Phase ii trial of neoadjuvant docetaxel and cg1940/cg8711 followed by radical prostatectomy in patients with high-risk clinically localized prostate cancer. Oncologist 2013, 18, 687–688.
- Ward, J.E.; McNeel, D.G., Gvax: An allogeneic, whole-cell, gm-csf-secreting cellular immunotherapy for the treatment of prostate cancer. Expert Opin. Biol. Ther. 2007, 7, 1893–1902.