Maternal milk, a complex fluid with several bioactive factors, is the best option for the newborn. Its dynamic composition is influenced by diverse factors such as maternal age, lactation period, and health status.
- adipokines
- antioxidants
- breastfeeding
- cytokines
- growth factors
1. Introduction
2. Bioactive Compounds in Breastmilk
There is wide evidence that BM prevents many of the perinatal complications associated with preterm labor [33], and BM is recognized as a protective factor against morbidity and mortality. Some of the beneficial factors against preterm complications may be related to the bioactive compounds and interactive elements present in the milk, such as antioxidants, growth factors, adipokines, cytokines, or antimicrobial compounds [34].2.1. Antioxidants in Human Milk
Reactive oxygen species (ROS) are physiologically relevant molecules that participate in cellular signaling processes. However, ROS are highly oxidizing and, in excess, can cause damage to cellular structures. To counteract the oxidative effects of ROS, there are a wide variety of antioxidant systems. There is a delicate balance between ROS and other reactive species and antioxidants. If this balance is lost, the result is oxidative stress. This can be due to an excessive ROS production that exceeds the antioxidant capacity, or insufficient antioxidant systems. Birth represents a significant oxidative challenge because of the switch from the relatively low-oxygen intrauterine environment to the high-oxygen extrauterine atmosphere [35]. Thus, newborns are exposed to an increase in ROS during labor and the transition to neonatal life [36]. PTB disrupts the normal developmental upregulation of antioxidant systems. Increased oxidative stress is observed in preterm neonates compared with full-term infants [37], being a critical factor that exacerbates perinatal morbidities of prematurity [38]. Endogenous antioxidants can be classified as enzymatic (i.e., superoxide dismutase (SOD), catalase, or glutathione peroxidase (GPx)), small non-enzymatic molecules (like glutathione (GSH)), or hormones with antioxidant capacity (such as melatonin) [37][39]. In addition to endogenous antioxidants, several foods, mainly vegetables and fruits, contain antioxidants such as vitamins, carotenoids, and polyphenols, among others.2.2. Growth Factors in Human Milk
Preterm infants are immature neonates who usually exhibit growth retardation, poor development and physical and neurological deficits. Postnatal growth retardation is likely the result of inadequate nutrition support after delivery, also contributing to poor neonatal health. In this context, in addition to a macronutrient supply, growth factors provided by breastfeeding may be of great importance. Growth factors play a role in the growth, maturation, and integrity of several organs, particularly for the neonatal gastrointestinal tract [40]. They help with the maturation of gut immunity and have anti-inflammatory effects [41][42]. Hirai et al. described the trophic effects of growth factors on fetal and neonatal gastrointestinal tract by promoting the proliferation and differentiation on their immature cells [43]. The highest concentrations of growth factors are provided by colostrum, as the first milk released after birth [44]. The main growth factors present in BM and their trophic effects on neonatal organs and systems are summarized in Figure 1.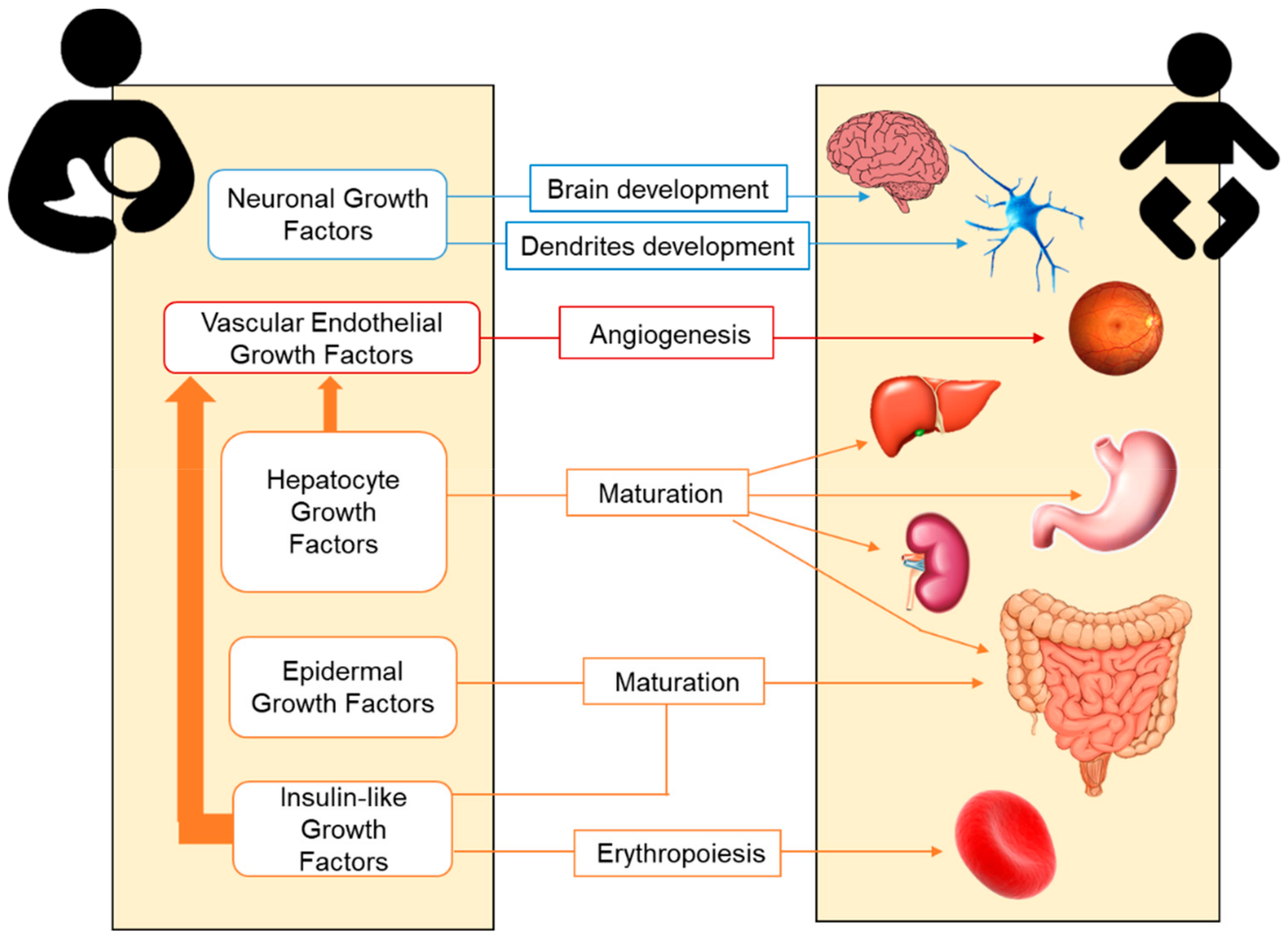
2.3. Adipokines in Human Milk
|
Adipokines |
Tissue Synthesized |
Range (ng/mL) |
Preterm Infants |
Main Neonatal Functions |
|---|---|---|---|---|
|
Leptin |
White adipose Placenta Mammary |
0.2–10.1 |
↑/? |
Anorexigenic T-lymphocyte responses |
|
Adiponectin |
Adipocytes |
4.2–87.9 |
≈/? |
Orexigenic Regulation of lipid/glucose metabolism Improvement of insulin sensitivity Anti-inflammatory actions |
|
Resistin |
Immune cells Epithelial cells |
0.2–1.8 |
↑/? |
Regulation of glucose homeostasis Inhibition of adipocyte differentiation Inflammatory response |
|
Ghrelin |
Stomach Pituitary Other |
0.07–6 |
? |
Orexigenic Gastric motility and secretion Adipogenesis Anti-inflammatory actions |
|
Obestatin |
Stomach Small intestine |
0.4–1.3 |
? |
Anorexigenic Body weight regulation |
|
Nesfatin |
Neurons Pancreas Other |
0.008–0.01 |
? |
Anorexigenic Production of body fat |
|
Apelin |
Heart Lung Other |
43–81 |
? |
Regulation of cardiovascular system Fluid homeostasis Angiogenesis Regulation of insulin secretion |
Adapted from Catli et al. (2014) [47]. Arrows indicate the comparison with term infants (↑, higher; ↓, lower; ≈, similar); ? indicates unavailable or inconclusive data and the need for research [47].
2.4. Cytokines in Human Milk
3. Human Milk Cells
3.1. Stem Cells in Human Milk
Recent data suggest that up to 6% of the cells in human milk are stem cells, and mesenchymal stem cells isolated from BM are potentially reprogrammable to multiple tissue types [56][57]. These cells may be involved in the development of immune cells including the regulatory T cell, which may produce tolerance to non-inherited maternal antigens and suppress anti-maternal immunity. It induces pregnancy microchimerism, leading to intestinal tissue repair and immune development and protection against infectious diseases [57].3.2. Leukocytes in Human Milk
Leukocytes are highly present in colostrum, which means that breastfed infants are exposed to up to 1010 maternal leukocytes/day [58]; however the contribution of this exposure in infants’ immune development is not yet clear [54]. A study carried out with 61 mothers and neonates showed a significantly smaller proportion of macrophages in BM of mothers with infants that developed an allergy to cow’s milk. In contrast, a higher content of neutrophils, eosinophils, or lymphocytes was associated with lower allergies to cow’s milk [59]. The role of these cells is still far from understood; for example, how these cells pass the stomach and intestinal barriers and access the infant, or their mechanisms of action, remain unclear. Therefore, further research to clarify these aspects of the inflammation and immunity development is required.4. Human Milk Microbiota
Early microbial colonization is essential for the infant’s metabolic and immunological maturation. Its development begins at birth, and the most important changes occur during the first year of life [60][61]. The microbiome is constantly changing, and it is influenced by hormones, cytokines, and chemokines. After birth, the transference continues along breastfeeding, and it is considered the main cause of variability between exclusively breastfed and formula-fed infants during the first months of life [56][62]. Raw BM is not a sterile food [58] and several reports confirm more than 100 types of viable bacteria/mL in human milk [62], including 65% of the phyla Proteobacteria and 34% of the phyla Firmicutes [49]. Regarding the genera, the most common are Staphylococcus, Streptococcus, Lactobacillus, Enterococcus, Lactococcus, Weissella, Veillonella, and Bifidobacterium [63][64]. It has been described that human milk microbiota at an early age is strongly linked with other perinatal factors such as place of residence, delivery mode, or maternal food intake [65]. With respect to the comparison between preterm and term milk, few studies have reported a difference in the BM microbiome. Some trends include more Bifidobacterium in term milk and more Enterococcus in preterm milk [52][58]. The association between the microbiome and various disorders, such as visceral pain, autism spectrum disorder, cardiovascular risk, obesity, depression, or multiple sclerosis, has been well demonstrated. Besides, the microbiota exerts immune-modulating effects that influence allergic reactions [60]. Thus, the gut microbiome from allergic children differs from non-allergic ones in composition and diversity [66]. It has been hypothesized that early gut colonizers could help in developing and maturing the immune system in infants [67]. Probiotic intake throughout gestation and lactation also leads to specific changes in the neonate [68]. A clinical trial reports the beneficial effect of oral supplementation with Lactobacillus rhamnosus in women during pregnancy and breastfeeding to reduce the allergy risk in infants. Modulatory effects were observed both on milk composition and the infant gut [69]. Variations in microbiota of preterm infants have been associated with a higher predisposition for developing NEC [70]. Taking together the information available, BM could be considered a probiotic food for infants. However, the potential protective effect of the BM microbiome is not fully understood, and additional research in this area is necessary to understand its role in the newborn’s health.References
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94.
- World Health Organization: Preterm Birth. Available online: https://bit.ly/2RWokG3 (accessed on 23 November 2018).
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.; Kinney, M. Born Too Soon: The Global Epidemiology of 15 Million Preterm Births. Reprod. Health 2013, 10, 1–14.
- Harrison, M.S.; Goldenberg, R.L. Global Burden of Prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79.
- Iams, J.D.; Romero, R.; Culhane, J.F.; Goldenberg, R.L. Primary, Secondary, and Tertiary Interventions to Reduce the Morbidity and Mortality of Preterm Birth. Lancet 2008, 371, 164–175.
- Ion, R.; López Bernal, A. Smoking and Preterm Birth. Reprod. Sci. 2015, 22, 918–926.
- Al-gubory, K. Environmental Pollutants and Lifestyle Factors Induce Oxidative Stress and Poor Prenatal Development. Reprod. Biomed. Online 2014, 29, 17–31.
- Simón, L.; Pastor-Barriuso, R.; Boldo, E.; Fernández-Cuenca, R.; Ortiz, C.; Linares, C.; Medrano, M.J.; Galán, I. Smoke-Free Legislation in Spain and Prematurity. Pediatrics 2017, 139, e20162068.
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, Regional, and Worldwide Estimates of Preterm Birth Rates in the Year 2010 with Time Trends since 1990 for Selected Countries: A Systematic Analysis and Implications. Lancet 2012, 379, 2162–2172.
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding Strategies for Premature Infants: Beneficial Outcomes of Feeding Fortified Human Milk Versus Preterm Formula. Pediatrics 1999, 103, 1150–1157.
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of Human Milk in Extremely Low Birth Weight Infants’ Risk of Necrotizing Enterocolitis or Death. J. Perinatol. 2009, 29, 57–62.
- Maayan-Metzger, A.; Avivi, S.; Schushan-Eisen, I.; Kuint, J. Human Milk Versus Formula Feeding Among Preterm Infants: Short-Term Outcomes. Am. J. Perinatol. 2012, 29, 121–126.
- Reiterer, F.; Scheuchenegger, A.; Resch, B.; Maurer-Fellbaum, A.; Avian, A.; Urlesberger, B. Outcomes of Very Preterm Infants with and without BPD Followed to Preschool Age. Pediatr. Int. 2019, 61, 381–387.
- Isaacs, E.B.; Fischl, B.R.; Quinn, B.T.; Chong, W.K.; Gadian, D.G.; Lucas, A. Impact of Breast Milk on Intelligence Quotient, Brain Size, and White Matter Development. Pediatr. Res. 2010, 67, 357–362.
- Parkinson, J.R.C.; Hyde, M.J.; Gale, C.; Santhakumaran, S.; Modi, N. Preterm Birth and the Metabolic Syndrome in Adult Life: A Systematic Review and Meta-Analysis. Pediatrics 2013, 131, e1240–e1263.
- Lapillonne, A.; Griffin, I.J. Feeding Preterm Infants Today for Later Metabolic and Cardiovascular Outcomes. J. Pediatr. 2013, 162, S7–S16.
- Howson, C.P.; Kinney, M.V.; Mcdougall, L.; Lawn, J.E. Born Too Soon: Preterm Birth Matters. Reprod. Health 2013, 10, 1–9.
- Castillo-Castañeda, P.C.; Gaxiola-Robles, R.; Méndez-Rodríguez, L.C.; Zenteno-Savín, T. Defensas Antioxidantes En Leche Materna En Relación Al Número De Gestas Y A La Edad De Las Madres. Nutr. Hosp. 2014, 30, 540–547.
- Castillo-Castañeda, P.C.; García-González, A.; Bencomo-Alvarez, A.E.; Barros-Nuñez, P.; Gaxiola-Robles, R.; Celina Méndez-Rodríguez, L.; Zenteno-Savín, T. Micronutrient Content and Antioxidant Enzyme Activities in Human Breast Milk. J. Trace Elem. Med. Biol. 2019, 51, 36–41.
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74.
- World Health Organization: Exclusive Breastfeeding. Available online: https://bit.ly/2FaaEkE (accessed on 23 November 2018).
- World Health Organization: Infant and Young Child Feeding. Available online: https://bit.ly/2VyOnRL (accessed on 5 March 2019).
- American Academy of Pediatrics. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841.
- Singhal, A.; Sadaf Farooqi, I.; O’Rahilly, S.; Cole, T.J.; Fewtrell, M.; Lucas, A. Early Nutrition and Leptin Concentrations in Later Life. Am. J. Clin. Nutr. 2002, 75, 993–999.
- Lessen, R.; Kavanagh, K. Practice Paper of the Academy of Nutrition and Dietetics Abstract: Promoting and Supporting Breastfeeding. J. Acad. Nutr. Diet. 2015, 115, 450.
- Yuksel, S.; Yigit, A.A.; Cinar, M.; Atmaca, N.; Onaran, Y. Oxidant and Antioxidant Status of Human Breast Milk During Lactation Period. Dairy Sci. Technol. 2015, 95, 295–302.
- Singhal, A.; Cole, T.J.; Lucas, A. Early Nutrition in Preterm Infants and Later Blood Pressure: Two Cohorts after Randomised Trials. Lancet 2001, 357, 413–419.
- Cubero, J.; Sánchez, C.L.; Bravo, R.; Sánchez, J.; Rodriguez, A.B.; Rivero, M.; Barriga, C. Analysis of the Antioxidant Activity in Human Milk, Day Vs. Night. Cell Membr. Free Radic. Res. 2009, 1, 100–101.
- De Vicente, B. 1 De Cada 5 Bebés no Recibe Leche Materna En Los Países Ricos. Available online: https://bit.ly/2RiYzeR (accessed on 21 January 2019).
- Heiman, H.; Schanler, R.J. Nutrición Enteral En Prematuros: El Rol De La Leche Humana. Rev. Enferm. 2007, 12, 26–34.
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients 2016, 8, 681.
- Mehta, R.; Petrova, A. Is Variation in Total Antioxidant Capacity of Human Milk Associated with Levels of Bio-Active Proteins? J. Perinatol. 2014, 34, 220–222.
- Savino, F.; Benetti, S.; Liguori, S.A.; Sorrenti, M.; Montezemolo, L.C. Di Advances on Human Milk Hormones and Protection Against Obesity. Cell. Mol. Biol. 2013, 59, 89–98.
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk—A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896.
- Mutinati, M.; Pantaleo, M.; Roncetti, M.; Piccinno, M.; Rizzo, A.; Sciorsci, R.L. Oxidative Stress in Neonatology: A Review. Reprod. Domest. Anim. 2014, 49, 7–16.
- Wilinska, M.; Borszewska-Kornacka, M.K.; Niemiec, T.; Jakiel, G. Oxidative Stress and Total Antioxidant Status in Term Newborns and Their Mothers. Ann. Agric. Environ. Med. 2015, 22, 736–740.
- Aceti, A.; Beghetti, I.; Martini, S.; Faldella, G.; Corvaglia, L. Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk. Oxid. Med. Cell. Longev. 2018, 2018, 1–7.
- Thibeault, D.W. The Precarious Antioxidant Defenses of the Preterm Infant. Am. J. Perinatol. 2000, 17, 167–182.
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants During Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175.
- Shelby, R.D.; Cromeens, B.; Rager, T.M.; Besner, G.E. Influence of Growth Factors on the Development of Necrotizing Enterocolitis. Clin. Perinatol. 2019, 46, 51–64.
- Lawrence, R.M.; Pane, C.A. Human Breast Milk: Current Concepts of Immunology and Infectious Diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2007, 37, 7–36.
- Loui, A.; Eilers, E.; Strauss, E.; Pohl-Schickinger, A.; Obladen, M.; Koehne, P. Vascular Endothelial Growth Factor (VEGF) and Soluble VEGF Receptor 1 (sFlt-1) Levels in Early and Mature Human Milk from Mothers of Preterm versus Term Infants. J. Hum. Lact. 2012, 28, 522–528.
- Hirai, C.; Ichiba, H.; Saito, M.; Shintaku, H.; Yamano, T.; Kusuda, S. Trophic Effect of Multiple Growth Factors in Amniotic Fluid or Human Milk on Cultured Human Fetal Small Intestinal Cells. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 524–528.
- Munblit, D.; Abrol, P.; Sheth, S.; Chow, L.Y.; Khaleva, E.; Asmanov, A.; Lauriola, S.; Padovani, E.M.; Comberiati, P.; Boner, A.L.; et al. Levels of Growth Factors and Iga in the Colostrum of Women from Burundi and Italy. Nutrients 2018, 10, 1216.
- Saso, A.; Blyuss, O.; Munblit, D.; Faal, A.; Moore, S.E.; Doare, K. Le Breast Milk Cytokines and Early Growth in Gambian Infants. Front. Pediatr. 2019, 6, 414.
- Savino, F.; Liguori, S.A.; Lupica, M.M. Adipokines in breast milk and preterm infants. Early Hum. Dev. 2010, 86, 77–80.
- Catli, G.; Anik, A.; Tuhan, H.Ü.; Kume, T.; Bober, E.; Abaci, A. The relation of leptin and soluble leptin receptor levels with metabolic and clinical parameters in obese and healthy children. Peptides 2014, 56, 72–76.
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508.
- Gregory, K.E.; Walker, W.A. Immunologic factors in human milk and disease prevention in the preterm infant. Curr. Pediatr. Rep. Online 2013, 1, 222–228.
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J. Hum. Lact. 2011, 27, 171–186.
- Watanabe, M.A.E.; de Oliveira, G.G.; Oda, J.M.M.; Ono, M.A.; Guembarovski, R.L. Cytokines in Human Breast Milk: Immunological Significance for Newborns. Curr. Nutr. Food Sci. 2012, 8, 2–7.
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014, 34, 599–605.
- Peroni, D.G.; Pescollderungg, L.; Piacentini, G.L.; Rigotti, E.; Maselli, M.; Watschinger, K.; Piazza, M.; Pigozzi, R.; Boner, A.L. Immune regulatory cytokines in the milk of lactating women from farming and urban environments. Pediatr. Allergy Immunol. 2010, 21, 977–982.
- Rajani, P.S.; Seppo, A.E.; Järvinen, K.M. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front. Pediatr. 2018, 6, 6.
- Polat, A.; Tunc, T.; Erdem, G.; Yerebasmaz, N.; Tas, A.; Beken, S.; Basbozkurt, G.; Saldir, M.; Zenciroglu, A.; Yaman, H.; et al. Interleukin-8 and its receptors in human milk from mothers of full-term and premature infants. Breastfeed. Med. 2016, 11, 247–251.
- Patki, S.; Kadam, S.; Chandra, V.; Bhonde, R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum. Cell 2010, 23, 35–40.
- Molès, J.P.; Tuaillon, E.; Kankasa, C.; Bedin, A.S.; Nagot, N.; Marchant, A.; McDermid, J.M.; Van de Perre, P. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol. 2018, 29, 133–143.
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8, 584.
- Järvinen, K.-M.; Soumalainen, H. Leucocytes in human milk and lymphocyte subsets in cow ’s milk-allergic infants. Pediatr. Allergy Immunol. 2002, 13, 243–254.
- Vass, R.A.; Kemeny, A.; Dergez, T.; Ertl, T.; Reglodi, D.; Jungling, A.; Tamas, A. Distribution of bioactive factors in human milk samples. Int. Breastfeed. J. 2019, 14, 1–10.
- Bendiks, M.; Kopp, M.V. The relationship between advances in understanding the microbiome and the maturing hygiene hypothesis. Curr. Allergy Asthma Rep. 2013, 13, 487–494.
- Perez, P.F.; Dore, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial Imprinting of the Neonatal Immune System: Lessons from Maternal Cells? Pediatrics 2007, 119, e724–e732.
- Mcguire, M.K.; Mcguire, M.A. Human Milk: Mother Nature’s Prototypical Probiotic Food? Adv. Nutr. 2015, 6, 112–123.
- Boix-Amorós, A.; Collado, M.C.; Mira, A. Relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Front. Microbiol. 2016, 7, 3389.
- Munblit, D.; Peroni, D.G.; Boix-Amorós, A.; Hsu, P.S.; Land, B.V.; Gay, M.C.L.; Kolotilina, A.; Skevaki, C.; Boyle, R.J.; Collado, M.C.; et al. Human Milk and Allergic Diseases: An Unsolved Puzzle. Nutrients 2017, 9, 894.
- Grönlund, M.; Gueimonde, M.; Laitinen, K.; Kociubinski, G.; Gronroos, T.; Salminen, S.; Isolauri, E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the bifidobacterium microbiota in infants at risk of allergic disease. Clin. Exp. Allergy 2007, 37, 1764–1772.
- Martín, V.; Maldonado-Barragán, A.; Moles, L.; Rodriguez-Baños, M.; Del Campo, R.; Fernández, L.; Rodríguez, J.M.; Jiménez, E. Sharing of Bacterial Strains Between Breast Milk and Infant Feces. J. Hum. Lact. 2012, 28, 36–44.
- Gueimonde, M.; Sakata, S.; Kalliomäki, M.; Isolauri, E.; Benno, Y.; Salminen, S. Effect of maternal consumption of Lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 166–170.
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 2016, 16, 1–14.
- Underwood, M.A.; Gaerlan, S.; De Leoz, M.L.A.; Dimapasoc, L.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677.
