Australia produces high-quality sweet cherries and generates revenue from local and export markets. Due to increased demand in the markets, the area of sweet cherry production has increased in Australia. Sweet cherry breeding and production have challenges such as self-incompatibility genotypes and phenotyping of agronomic, physiological, and quality traits. Phenotyping is identified as an interaction between cultivars and plant responses to different environmental conditions which determine the plant yield and fruit quality. Efficient phenotyping techniques are important to develop new sweet cherry cultivars in Australia with high yield potential, high fruit quality, temperature tolerance (cold and heat), and drought tolerance.
- environmental factors
- fruiting spur
- fruit development
1.1. Agronomic Traits
The important agronomic traits of sweet cherry are blooming and fruit development and have a direct influence on maturity [1][8]. Fruits from early and late-maturity cultivars attract a higher price on the market [2][9]. For both maturity groups, the periods of bud breaking, blooming, and maturity are important traits in sweet cherry production [3][10]. The positive correlation between blooming and maturity period has been observed in peaches and apricots [4][5][11,12]. Breeders need knowledge of the blooming and fruit development for various sweet cherry germplasm and the impact of cold and high temperatures during that period. Sweet cherry has three stages of fruit development (double sigmoid growth pattern). In Stage 1, cell division and enlargement of the pericarp occurs. In Stage 2, embryo development occurs, which causes pit hardening. Usually, Stage 2 is shorter in early cultivars than late cultivars and has a high chance for abortion [6][13]. Cold temperature during Stage 2 in early cultivars was the main reason for embryo abortion [7][14]. Day temperature ≤ 16 °C had an adverse effect on embryo development in sweet cherry [8][15]. The pit hardening stage was also susceptible to low temperatures in stone fruits [9][16]. Particularly, sensitivity of temperature in sweet cherry endures the transition period from Stage 1 to Stage 2 and temperature also had an impact on fruit quality [8][15]. Fruit development was studied from peak blooming to fruit maturity using cumulative growing degree days (CGDD) [10][17]. The base temperature for the peak blooming period was 4 °C. For example, the most popular mid-season cultivar, Bing, needs ≥ 800 chilling hours and 683 positive chilling units during 30% bud breaking period in Orange (NSW), Australia. This indicates that the Bing cultivar has a high chilling requirement in Stage 1 [11][18]. Optimum (24 °C) and critical temperatures (>35 °C) for cherry fruit development were also identified [10][17]. Duration of Stage 2 was increased due to cold temperatures (4 °C to 15 °C) for late-maturing cultivars [10][17]. The critical temperature of >35 °C during fruit development for sweet cherry is similar to seed development in grain crops such as wheat [12][19], chickpea [13][20], and peanuts [14][21]. Stage 3 of cherry fruit development consists of continuation of fruit growth and ripening of the exocarp (fruit flesh). It is a critical period to achieve high yield and fruit quality. The optimum temperature during Stage 1 and Stage 2 fruit development has a major influence on Stage 3 fruit development [8][15]. Hence, temperature is a key factor influencing flowering and fruit development. Rootstocks regulate the subsequent tree growth, management of the orchard, and crop yield. It is also important that the selection of rootstock has to match the environmental condition of the orchard [15][2]. In Australia, Mazzard rootstocks are compatible with most sweet cherry cultivars and the Mahaleb rootstock is incompatible with Australian sweet cherry cultivars [15][2]. The influence of rootstock on the sweet cherry cultivar affects the timing of flowering, the intensity of flowering, fruit quality, and yield [16][22]. The influence of rootstock on the flower bud sensitivity to frost (−5 °C to −7 °C) was studied in Belgrade. Rootstock affected the flower bud blooming time, flowers per bud, timing of harvest, fruit weight, and yield [16][22]. Rootstocks also influence the number of flowers and percentage of fruit set. However, certain cultivars had impaired pollen viability and pollen germination under frost [17][23]. For example, the ‘Kordia’ cultivar had small pollen grains and poor viability. The cultivar ‘Regina’ had more frost resistance than ‘Kordia’ [16][22]. These cultivars have a great impact on the selection of rootstock in the breeding programme. Therefore, the rootstocks influence the performance of the sweet cherry cultivars and have an effect on tree growth, crop yield, flowering, and pollination. Though the influence of rootstocks on the cultivar is mentioned in this section, the importance of rootstocks in sweet cherry breeding is discussed in greater detail in Section 5.1.2. Physiological Traits
Australian sweet cherry tree growth stages and physiological process are outlined in Figure 1. Each stage is influenced by tree density, genetic material, temperature, and water availability. The key physiological drivers for fruit growth in sweet cherry are photosynthesis and carbon–nitrogen mobilisation of tissues. Different tree planting systems are being used commercially in Australia. Simplified tree structures encourage light penetration and photosynthesis. Therefore, leaf traits are important physiological traits in sweet cherry breeding. The leaf length and width had a positive correlation with fruit weight in sweet cherry [18][24]. Consequently, the role of photosynthesis and leaf size (length and width) are important in determining fruit size [19][20][25,26]. Higher photosynthetic productivity and leaf size could be achieved with higher light interception in the sweet cherry tree [21][27].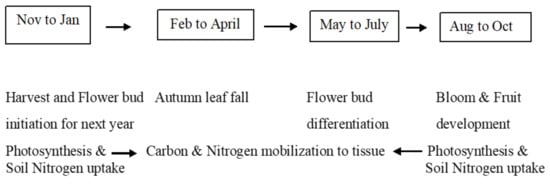
1.3. Impact of Environmental Factors in Agronomic, Physiological and Reproductive Traits
Generally, each stage in fruit development is affected by temperature. Flower bud differentiation was slow when average temperatures reached above 18 °C, which indirectly caused shorter fruit development periods [30][36]. Temperature has a direct influence on the period of fruit development on Stage 1. Low temperature (<12 °C) combined with rain negatively influenced the pollination and activity of bees and fruit set [31][32][37,38]. Generally, honey bees are active pollinators at or above 19 °C and completely cease their activity at <13 °C. Wind speeds at >8 km/h and rain also stop honey bee activity. In Tasmania, cherry growers use bumblebees as a pollinator to achieve effective pollination because bumblebees are active under cold and wet conditions [15][2]. Five bee species that are active at low temperatures (≤12 °C) combined with rainy days were identified in Turkey [31][37]. Hence, there are potential alternative pollinators which can be active in adverse climatic conditions to achieve economic returns from the cherry orchard. Simultaneously, high temperatures (>30 °C) during flowering time, particularly the differentiation of pistil primordia (stigma, style, and ovary) created double fruits [32][33][38,39]. High temperatures (29 to 35 °C) during the harvest period caused an increase in the number of double fruits [34][40]. Furthermore, high temperatures accelerate pollen hydration and ovule senescence in sweet cherry [35][41]. Therefore, extreme temperatures (too cold—>0 °C and too hot—≥35 °C) have a negative impact on reproductive organs in sweet cherry and reduce crop yield by shortening the period of fruit development. Another factor which influences agronomic and physiological traits in sweet cherry production is water supply. Most Australian cherry production areas have limited water supply and need careful water management in the cherry orchards [15][2]. Generally, the harvest period for sweet cherries in Australia relies on a warm summer with less rain. In the fruiting period, the tree continually develops leaves and flower buds for the following season (Figure 1). It is common for fruit growers to apply water during the harvest period to match summer temperatures. The amount of water supply at this stage depends on crop age, planting density, and climatic conditions of the orchard. For example, the average daily water uptake of the 4 years old trees (early cultivar ‘Rita’) in a high-density orchard (spacing 4 m × 2 m) during summer (May to July) in Hungary was 23–24 L per tree [36][42]. Water stress (where tree demand exceeds the supply) at harvest can cause interruption in photosynthesis, leading to changes in biochemical processes in leaves such as leaf sugar content [37][43]. Regulated deficit irrigation (RDI) 65% of crop evapotranspiration during harvest was monitored by stem water potential rather than fruit water potential. RDI (65% of crop evapotranspiration) during harvest did not affect the current year’s fruit yield and quality [38][44]. Irrigation with 85% of crop evapotranspiration during preharvest and 55% of crop evapotranspiration at postharvest did not affect flowering, fruit set, and fruit growth [34][40]. However, irrigation during preharvest and postharvest periods which did not meet plant water requirements decreased vegetative growth and leaf area: fruit ratio, which subsequently affects fruit size. Consequently, regulated deficit irrigation which meets plant water requirements in critical periods of flowering and fruit development saved up to 40% irrigation water without decreasing fruit yield [39][45]. Sweet cherry phytochemicals (total sugars, total organic acids, and anthocyanins) in fruits were higher in irrigated trees (rain water plus 100% evapotranspiration) than in non-irrigated trees (only rain water) [40][46]. Therefore, adequate chilling hours during blooming, optimum temperatures during fruit development, water supply based on tree demand during flowering, and fruit development encourage good sweet cherry fruit quality and crop yield.2. Phenotyping Based on Fruit Quality Traits in Sweet Cherries
From the commercial point of view, the key sweet cherry fruit quality traits are fruit size, fruit colour, firmness, and sweetness [24][41][42][30,47,48]. The Australian cherry production guide explains the quality descriptions for fruit size, fruit colour, sweetness, and firmness tester. The fruit size varies from 22 mm to 32 mm (in diameter) (Figure 2). The price of cherries varies with size, along with its firmness, fruit colour, and sweetness. Fruit firmness is an important trait in cherry quality, which is usually tested by fruit firmness tester (or) penetrometer and expressed as g/m2. Fruit sweetness is determined by soluble solid concentration using a digital refractometer (% brix). The fruit firmness and sweetness by commercial standards from the Australian cherry production guide is explained in Table 1.
| Firmness | g/m2 | Sweetness | Brix % |
|---|---|---|---|
| Hard | >350 | Super sweet | ≥21 |
| Very firm | 300–350 | Very sweet | 19–20 |
| Firm | 240–300 | Sweet | 17–18 |
| Less firm | <240 | Moderate sweet | 16 |
