Quercetin is a flavonoid, present in various natural sources, which has demonstrated in vitro and in vivo antidiabetic properties. It improves oral glucose tolerance, as well as pancreatic β-cell function to secrete insulin. It inhibits the α-glucosidase and DPP-IV enzymes, which prolong the half-life of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Quercetin also suppresses the release of pro-inflammatory markers such as IL-1β, IL-4, IL-6, and TNF-α.
- quercetin
- diabetes
- inflammatory markers
- medicinal plants
- insulin
1. Introduction
Diabetes mellitus (DM) is a chronic disease that is one of the leading causes of illness and mortality across the globe. DM is diagnosed as a result of an elevated blood glucose level (hyperglycaemia) caused by inadequate insulin secretion, defective insulin action, or both. The improper control of insulin has also been linked to abnormalities in the metabolism of lipids and proteins. If proper treatment is not received on time, or if left untreated, DM can lead to hyperglycaemic coma, and severe damage to the eyes, kidneys, blood vessels, and nervous and cardiovascular system. It can even lead to death due to ketoacidosis and nonketotic hyperosmolar syndrome [1][2][1,2]. These metabolic disruptions result from low insulin levels or insulin resistance in skeletal muscles, adipose tissue, and other target tissues. The development, pathogenesis, and complications of DM have been strongly correlated with high levels of oxidative stress, free radicals, and other metabolic stressors [3][4][3,4]. According to reports from 2021, 465 million people suffer from DM worldwide [5]. This number is anticipated to rise to 700 million by 2045. The majority of DM sufferers are from middle and low-income countries [5].
The American Diabetes Association has categorized diabetes as Type 1, Type 2, and gestational DM [6]. Type 1 diabetes, also known as juvenile diabetes, causes a decrease in glucose sensitivity to clonal pancreatic β-cells [7]. It has no cure but can be controlled by lifestyle changes, blood sugar monitoring, and the administration of insulin. This type of diabetes occurs in approximately 80–90% of children and adolescents [8]. Type 2 diabetes mellitus (T2DM) is the most prevalent and occurs due to the insufficient production of insulin by the body, insulin resistance, and obesity [9]. It can be controlled by lifestyle/dietary changes and oral antidiabetic drugs but requires insulin in severe cases [10]. Whilst the majority of T2DM sufferers are adults (more than 90% of the patient population), it affects people of all ages. Individuals over 40 years of age and with obesity issues and a family history of the disease are at a higher risk of developing T2DM [11].
A range of antidiabetic drugs such as metformin, sulfonylureas, meglitinides, thiazolidinediones, GLP-1 mimetics, DPP-IV, and SGLT2 inhibitors are currently used to treat T2DM. However, many of these are costly and present notable adverse side effects (Table 1) [12].
Table 1.
Pharmacological actions and side effects of antidiabetic drugs.
|
Type 2 Antidiabetic Agents |
Pharmacological Actions |
Side Effects |
References |
|
α-glucosidase inhibitors (Acarbose, miglitol) |
Inhibit the intestinal absorption of carbohydrates |
Flatulence, bloating, diarrhoea |
[20,21] |
|
Biguanides (Metformin) |
Inhibit hepatic gluconeogenesis, Reduce the liver and intestinal absorption of sugar Increase insulin sensitivity and glucose uptake |
Kidney complications, upset stomach, tiredness, and dizziness |
[22,23] |
|
Dopamine agonists (Bromocriptine, cabergoline, apomorphine) |
Regulate plasma glucose, free fatty acids, and triglyceride levels in insulin-resistant patients |
Visual hallucinations and confusion, edema |
[24,25] |
|
Dipeptidyl peptidase-4 (DPP-4) inhibitors (Sitagliptin, saxagliptin, linagliptin) |
Increase the half-life of GLP-1 and GIP |
Gastrointestinal problems, flu-like symptoms (headache, runny nose, sore throat) |
[26,27] |
|
GLP-1 agonists (Dulaglutide, exenatide, albiglutide) |
Enhance insulin release Reduce glucagon release |
Gastrointestinal problems and nausea |
[28,29] |
|
Meglitinides (Nateglinide, repaglinide) |
Stimulate the release of insulin |
Weight gain, hypoglycaemia, excessive sweating |
[30,31] |
|
Sodium-glucose Co-transporter-2 (SGLT-2) inhibitors (Dapagliflozin, canagliflozin, empagliflozin) |
Inhibit glucose reabsorption in the renal tubule |
Urinary tract infection and increased urination, upper respiratory tract infections, joint pain, nausea, and thirst |
[32,33] |
|
Sulfonylureas (Tolbutamide, tolazamide, chlorpropamide) |
Inhibit ATP-sensitive potassium (KATP) channel in pancreatic β-cells |
Hypoglycaemia, upset stomach, skin rash, and itching |
[34] |
|
Thiazolidinediones (Rosiglitazone, pioglitazone) |
Bind with the peroxisome proliferator-activated receptor (PPAR)-γ receptor resulting in the activation of several genes that regulate glucose metabolism in the liver |
Anaemia risk, weight gain, edema, heart failure |
[35,36] |
|
α-glucosidase inhibitors (Acarbose, miglitol) |
Inhibit the intestinal absorption of carbohydrates |
Flatulence, bloating, diarrhoea |
|
|
Biguanides (Metformin) |
Inhibit hepatic gluconeogenesis, Reduce the liver and intestinal absorption of sugar Increase insulin sensitivity and glucose uptake |
Kidney complications, upset stomach, tiredness, and dizziness |
|
|
Dopamine agonists (Bromocriptine, cabergoline, apomorphine) |
Regulate plasma glucose, free fatty acids, and triglyceride levels in insulin-resistant patients |
Visual hallucinations and confusion, edema |
|
|
Dipeptidyl peptidase-4 (DPP-4) inhibitors (Sitagliptin, saxagliptin, linagliptin) |
Increase the half-life of GLP-1 and GIP |
Gastrointestinal problems, flu-like symptoms (headache, runny nose, sore throat) |
|
|
GLP-1 agonists (Dulaglutide, exenatide, albiglutide) |
Enhance insulin release Reduce glucagon release |
Gastrointestinal problems and nausea |
|
|
Meglitinides (Nateglinide, repaglinide) |
Stimulate the release of insulin |
Weight gain, hypoglycaemia, excessive sweating |
|
|
Sodium-glucose Co-transporter-2 (SGLT-2) inhibitors (Dapagliflozin, canagliflozin, empagliflozin) |
Inhibit glucose reabsorption in the renal tubule |
Urinary tract infection and increased urination, upper respiratory tract infections, joint pain, nausea, and thirst |
|
|
Sulfonylureas (Tolbutamide, tolazamide, chlorpropamide) |
Inhibit ATP-sensitive potassium (KATP) channel in pancreatic β-cells |
Hypoglycaemia, upset stomach, skin rash, and itching |
[27] |
|
Thiazolidinediones (Rosiglitazone, pioglitazone) |
Bind with the peroxisome proliferator-activated receptor (PPAR)-γ receptor resulting in the activation of several genes that regulate glucose metabolism in the liver |
Anaemia risk, weight gain, edema, heart failure |
2. Chemistry of Quercetin
The term quercetin is derived from the Latin word “Quercetum” which means oak forest. The main dietary sources of quercetin are fruits, vegetables, and various medicinal plants. Quercetin (3, 3′,4′,5, 4′, 5, 7-pentahydroxyflavone) is a compound yellow in color, fully soluble in lipids and alcohol, insoluble in cold water, and sparingly soluble in hot water, that was isolated as a flavonoid glycoside for the first time in 1854. Its chemical structure was elucidated in 1899 [30][37]. Quercetin belongs to the flavonol subclass of flavonoids, with two aromatic rings (A and B) interlinked by a three-carbon linked γ-pyrone ring (C), and five hydroxyl (OH) groups that can be variously substituted (Figure 1).
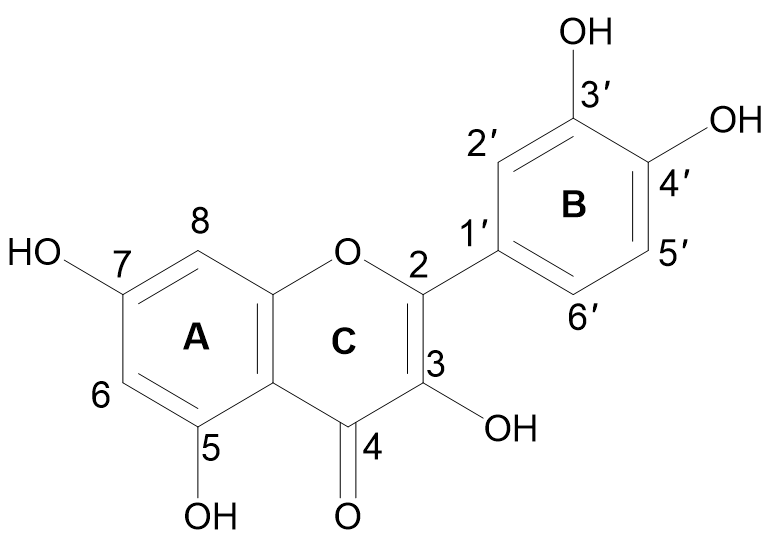
Figure 1.
Chemical structure of quercetin with (A, B) and (C) representing the aromatic and γ-pyrone rings, respectively.
The majority of quercetin derivatives are found in a glycoside form in which one or more hydroxyl group is substituted by different types of sugars [31][38]. Its polyphenolic structure, catechol moiety in the B ring, OH groups at positions 3 and 5 in the A ring, and 2,3-double bond conjugated with a 4-oxo function in the C ring have been identified as important features responsible for the well-known antioxidant effect of quercetin [32][33][39,40].
3. Pharmacological Actions of Quercetin in Diabetes and Associated Metabolic Disorders
Quercetin possesses various pharmacological properties and has been reported as one of the most widely used flavonoids to treat metabolic and inflammatory disorders [34][14]. In vitro studies on human retinal endothelial cells demonstrated that quercetin could inhibit the proliferation of high-glucose-induced cells by lowering the production of vascular endothelial growth factor (VEGF) (Figure 2) [35][41]. Quercetin also inhibited carbohydrate digesting-enzymes (intestinal α-glucosidase and pancreatic α-amylase), reduced starch hydrolysis, decreased the rate of glucose absorption, as well as slowed down the progression of postprandial hyperglycaemia in in vitro settings (Figure 2) [36][37][42,43].
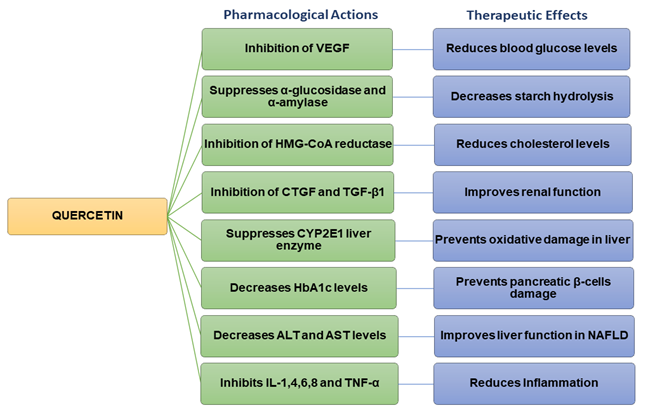
Figure 2.
Flow chart summarizing the pharmacological actions and therapeutic effects of quercetin.
Studies carried out on streptozotocin (STZ)-induced diabetic rats have revealed that quercetin could reduce blood glucose levels and improve glucose tolerance [38][44]. Quercetin decreased plasma glucose levels in Type 2 diabetic rats [39][45]. In hyperlipidaemic animals, quercetin lowered the levels of triglycerides (TG), total cholesterol (TC), LDL, and VLDL cholesterol, inhibited 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, and increased adiponectin and HDL cholesterol levels [39][40] [45,46]. Previous findings also indicated that quercetin could improve the high-fat diet (HFD)-induced dyslipidaemia in Swiss albino mice [41][47]. Other studies have shown that quercetin inhibited the overexpression of connective tissue growth factor (CTGF) and transforming growth factor beta-1 (TGF-β1) and contributed to improving renal function in diabetic nephropathic rats (Figure 2) [42][48].
Quercetin has the potential to prevent diabetic liver oxidative damage by suppressing the CYP2E1 liver enzyme in diabetic mice (Figure 2) [49]. Additionally, it decreases oxidative stress in diabetic renal tissue [50]. The administration of quercetin decreased body weight, fat accumulation, hyperglycaemia, dyslipidemia, and hyperinsulinemia in high-fat-fed obese mice [50]. Quercetin reduced blood glucose levels in mice and rats with T2DM [45,51]. It also decreased oxidative damage in the pancreatic tissue of high-fat-fed mice [52]. When combined with resveratrol, quercetin significantly upregulated gene-associated glucose or lipid metabolism, as well as liver function, in HFD animal models [53]. Furthermore, quercetin with/without resveratrol reduced the damage to pancreatic β-cells by restoring serum C-peptide and glycosylated hemoglobin (HbA1c) levels in diabetic rats (Figure 2) [54]. Histological investigations demonstrated that quercetin, with/without resveratrol, preserved pancreatic tissue and regulated insulin levels, thereby exerting hypoglycemic activity, and enhancing the function of pancreatic β-cells in diabetic rats [55]. Quercetin reduced serum lipids levels and showed beneficial effects on dyslipidemia-associated complications including atherosclerosis, myocardial attack, and coronary diseases [50]. Quercetin significantly decreased plasma glucose levels in STZ-induced diabetic rats. In addition, it improved glucose tolerance and hepatic glucokinase activity [44]. Quercetin increased the number of pancreatic islets in both normal and diabetic mice. It also regenerated the pancreatic islets and enhanced insulin secretion in STZ/alloxan-induced diabetic mice [56].
In a randomized, double-blind, placebo-controlled clinical trial, quercetin at a dose of 100 mg/day for 12 weeks reduced body fat and body mass index (BMI) of obese subjects [57]. It also downregulated triacylglycerol levels at a dose of 150 mg/day in overweight individuals [57]. Quercetin also lowered maltose-induced postprandial hyperglycaemia but had no significant effect on glucose-induced postprandial hyperglycaemia [58]. The oral administration of multiple doses of quercetin decreased blood glucose and HbA1c levels, enhanced glycogen synthesis, decreased α-glucosidase activity, and insulin resistance. In addition, it minimized β-cell insufficiency, enhancing pancreatic insulin secretion and controlling blood glucose levels in diabetic patients by reducing oxidative stress [59].
4. Mechanisms of Action of Quercetin
Quercetin maintains glucose homeostasis by interacting with molecular targets in the small intestine, pancreas, skeletal muscle, adipose tissue, and liver. Studies carried out on STZ-induced diabetic rats have revealed that quercetin could restore the impaired protein expression of insulin signaling molecules, such as phosphatidylinositol 3 kinases (PI3K) and insulin receptor substrate-1 (IRS-1), resulting in increased insulin-mediated glucose uptake [54][117]. Quercetin has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK) in the livers of rats, which reduces glucose synthesis primarily via downregulating glycogenic isoenzymes, such as phosphoenolpyruvate carboxylase (PEPCK) and glucose-6-phosphatase (G6Pase) [46][55][52,118]. In mouse skeletal muscle cells, it has been reported to enhance glucose uptake by promoting the translocation of GLUT4 to the cell membrane [56][119]. These findings indicate that quercetin regulates the metabolism of glucose, increasing glycolysis while decreasing gluconeogenesis [57][120]. In healthy individuals, around 80% of the absorbed glucose is stored in the form of glycogen in skeletal muscles upon the action of insulin. A reduction in this uptake has been shown to contribute to the etiology of T2DM as irregularities in the expression of the GLUT4 transporter lower the rate of glucose entering the cells, leading to a rise in blood glucose levels [58][121]. In skeletal muscles, quercetin activates AMPK, which in turn stimulates GLUT4 receptors and Akt (protein kinase B) in the cell membrane [59][122]. This allows glucose to enter the cells via the GLUT4 transporter, thereby regulating glycaemia [54][117]. Similarly, exercise is a potent activator of GLUT4 expression, which increases insulin activity and muscle glycogen storage. Defective activation of AMPK leads to insulin resistance, which causes T2DM [60][123]. Quercetin-induced AMPK activation in hepatocytes inhibits glucose-6 phosphatases [55][118]. Treatment with quercetin decreases GLUT2 expression and the intestinal sodium-dependent glucose uptake, in turn reducing glucose absorption in the gastrointestinal tract and controlling glycaemia (Figure 3) [56].

Figure 3. Pharmacological action of quercetin via different mechanistic pathways: Quercetin enhances pancreatic β-cell function and increases insulin release by inhibiting apoptosis, NF-κB, and JNK pathways; decreases glucose absorption in the kidney by inhibiting DPP-IV and COX-2 activity; decreases gluconeogenesis through inhibition of TNF-α and IL-4 in the liver; suppresses glucose reabsorption in the gastrointestinal tract by decreasing α-glucosidase activity; reduces blood glucose levels and oxidative stress by inhibiting IL-6 activity in the heart and blood vesselsgure 3) [119].
In addition, quercetin has been reported to enhance the AMP/ATP ratio and scavenge reactive oxygen species (ROS) in clonal pancreatic β-cells, reducing oxidative stress (Figure 3) [61][124]. The increased AMP/ATP ratio activates the mitochondrial target of rapamycin (mTOR), which induces mitogenesis (via transduction signaling pathways activation) and stimulates insulin secretion [62][125]. mTOR plays a significant role in the regulation of transcription, protein synthesis, and cell nutrition [63][126]. Under hyperglycaemic conditions, proteins, lipids, and nucleic acids undergo non-enzymatic glycation to form Advanced Glycation End (AGE)-products. The latter cause diabetic complications such as cardiovascular diseases, nephropathy, retinopathy, and neuropathy. Quercetin has been found to inhibit protein glycation more potently than the synthetic drug aminoguanidine [59][60][122,123]. It can reduce the formation of AGE-products by trapping methylglyoxal and glyoxal [64][127] and improve diabetic complications due to its antioxidant, anti-inflammatory, and antihyperglycemic properties [41][47].
In STZ-induced rats, quercetin improved retinopathy by down-regulating matrix metalloproteinase-9 (MMP-9), monocyte chemo-attractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF) [128]. In hypercholesterolemic mice, it reduced diabetic nephropathy by lowering triglycerides and blood glucose levels [129]. Moreover, it also showed neuroprotective effects on enteric neurons in the cecum of DM rats [130].
Previous studies have revealed that fat accumulation in the liver and muscles activates the Jun N-terminal kinases (JNK) and the nuclear transcription factor Kappa-B (NF-κB) inflammatory pathways, leading to obesity-associated T2DM (Figure 3) [131]. Quercetin suppresses both these pathways, which in turn improves glycaemia [132]. It also suppresses the FcεRI receptor by inhibiting the phosphorylation of several kinases like PKC (protein kinase C), Syk (spleen tyrosine kinase), and p38 mitogen-activated protein kinase (MAPK) in mast cells and basophils [122].
The release of pro-inflammatory mediators such as IL-1, IL-6, IL-8, IL-4, TNF-α, and histamine in brown adipose tissue has been linked with increased insulin resistance and high blood glucose levels (Figure 3) [70][133]. Quercetin inhibits these mediators and prevents oxidative stress [71][134]. In the kidneys, quercetin reduces DPP-IV and cyclooxygenase-2 (COX-2) activity, leading to a decrease in blood glucose reabsorption (Figure 3) [72][135]. Quercetin also activates leukocytes and targets various enzymes such as kinases, membrane proteins, and phosphatases to control inflammation and the immune response [66][129]. It suppresses lipoxygenase and cyclooxygenase enzymes, which suppresses the release of pro-inflammatory mediators including leukotrienes and prostaglandins [73][136]. Quercetin also inhibits TNF-α, a cytokine that plays a vital role in leukocyte formation, proliferation, and differentiation, specifically in the liver and gastrointestinal tract [62][72][74][69,125,135]. This causes a reduction in gluconeogenesis, glucose reabsorption, and α-glucosidase activity (Figure 3) [72][135]. Pancreatic β-cell apoptosis may occur due to hyperglycaemia-induced oxidative stress, and this can lead to diabetes mellitus. Glutathione peroxidase 4 (GPX4), an enzyme that protects cells against lipid peroxidation, suppresses the ferroptosis or apoptosis of pancreatic β-cells [75][137]. It has been demonstrated that quercetin can increase GPX4 activity in the pancreas, reducing oxidative stress, increased β-cell production, and insulin secretion [76][138]. Quercetin has also been reported to reduce the intestinal absorption of cholesterol by reducing the expression of the epithelial cholesterol transporter Niemann-Pick C1-Like 1 (NPC1L1) [77][100] and it has been suggested that the consumption of quercetin in the diet could lower systolic, diastolic, and mean arterial pressure in hypertensive individuals [78][139]. Quercetin can also lower blood pressure by reducing oxidative stress, enhancing the renin-angiotensin-aldosterone system (RAAS), and increasing vascular activity [70][133].
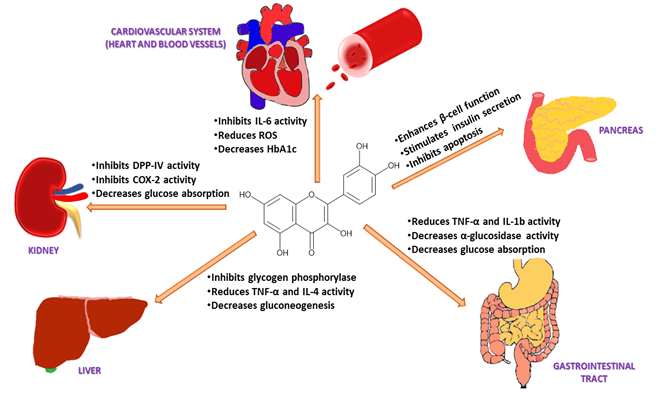
Figure 3. Pharmacological action of quercetin via different mechanistic pathways: Quercetin enhances pancreatic β-cell function and increases insulin release by inhibiting apoptosis, NF-κB, and JNK pathways; decreases glucose absorption in the kidney by inhibiting DPP-IV and COX-2 activity; decreases gluconeogenesis through inhibition of TNF-α and IL-4 in the liver; suppresses glucose reabsorption in the gastrointestinal tract by decreasing α-glucosidase activity; reduces blood glucose levels and oxidative stress by inhibiting IL-6 activity in the heart and blood vessels.
