Chemotherapy is one of the standard methods for the clinical treatment of malignant tumors. Due to the heterogeneity of tumors and the complexity of their pathological mechanisms, a single chemotherapeutic drug is usually unable to eradicate cancer cells. It may also encounter some problems, such as toxic side effects induced by high doses of drugs and obtaining multidrug resistance (MDR) after repeated treatment. These problems then increase the likelihood of cancer metastasis or recurrence. The emergence of the combination of multiple antineoplastic drugs makes up for the deficiency of single drug application. Accordingly, the overall treatment benefit of the multidrug combination is usually higher than that of single drug administration by virtue of different therapeutic mechanisms. More importantly, the drug dose used during synergistic therapy usually decreases and the unfavorable side effects could be weakened under the premise of the same or better therapeutic efficacy. Thereinto, conjugated nanomedicine, as an important type of nanomedicine, can not only possess the targeted delivery of chemotherapeutics with great precision but also achieve controlled drug release to avoid adverse effects. Meanwhile, conjugated nanomedicine provides the platform for combining several different therapeutic approaches (chemotherapy, photothermal therapy, photodynamic therapy, thermodynamic therapy, immunotherapy, etc.) with the purpose of achieving synergistic effects during cancer treatment. Therefore, this review focuses on conjugated nanomedicine and its various applications in synergistic chemotherapy.
1. Synergistic Chemo-Chemo Therapy
Different types of chemotherapeutic drugs can be classified according to their function and mechanism of action on cancer cells. The most common classes of chemotherapeutic drugs are alkylating agents, antimetabolic agents, anthracyclines, topoisomerase inhibitors, mitotic inhibitors, and corticosteroids. The choice of two or more chemotherapeutic drugs depends on the stage and type of cancer, the synergistic behaviors of various drugs and other factors. The choice of drugs determines whether the effect is synergistic, additive, or antagonistic
[1][149]. Conventional “cocktail” treatment generally presents an inadequate enhancement of therapeutic efficiency since different anticancer drugs often display diverse pharmacokinetics and transmembrane ability, resulting in the uncontrollable distribution of drugs in tumors
[2][3][4][150,151,152]. To overcome the above challenges, nano-carrier based multiple drugs co-delivery systems have been developed for cancer synergistic chemo–chemo therapy, which are capable of delivering two or more chemotherapeutics to tumors in a desirable ratio by virtue of the advantages of nanomedicine. Once connected, the hydrophobic and hydrophilic anti-tumor drugs, serving as building blocks, could also provide the impetus to fabricate the nanoparticles. This kind of conjugated nanomedicine not only displays the advantages of high drug loading content but also relatively stable drug delivery capacity, reduced side effects and enhanced anticancer activity benefitting from the synergistic effect of different drugs
[5][6][7][153,154,155]. Previously,
thwe
researchers hhave introduced the conjugated nanomedicine (denoted as MTX-SS-PPT NAs) developed by Hou and co-workers, which is self-assembled from the drug conjugates containing hydrophilic drug
methotrexate (MTX)MTX and the hydrophobic drug P
odophyllotoxin (PPT)PT. In this nanomedicine, the disulfide bonds connecting the two drugs contribute to the degradation of MTX-SS-PPT NAs in tumor cells under reduction conditions. As a slow-release of the active drug, the nanoagent can significantly improve the biocompatibility of PPT and reduce its toxicity
[8][98].
The development and metastasis of solid tumors highly depend on the formation of neovascularization. However, the use of angiogenesis inhibitors alone cannot meet the needs of cancer treatment. Sun and co-workers conjugated hydrophilic chemotherapeutic drugs (fluorosarboside, FUDR) with hydrophobic antiangiogenic drugs (pseudoboric acid B, PAB), followed by formulating nanoparticles in an aqueous solution. These nanoparticles not only displayed promising anti-tumor activity but also had efficient antiangiogenesis properties, leading to a good cancer therapeutic outcome in mice bearing subcutaneous HeLa tumors
[9][156]. Dasatinib (DAS) is a competitive oral dual Src/Abl kinase inhibitor, which can inhibit a variety of Src signal pathways and further inhibit tumor cell migration, invasion and angiogenesis
[10][157]. Yang and co-workers linked DAS with cisplatin octahedral coordination derivative diamino dichlorodihydroxyplatinum (DH-CP) through an ester bond to form an amphiphilic drug–drug conjugate (CP–DDA) at the ratio of 2:1. Then, the stable nanoparticles (CP–DDA NPs) were formed by the self-assembly of CP–DDA in an aqueous solution. The nanoparticles displayed promising stability during blood circulation and increased accumulation of drugs in the tumor site through the
enhanced permeability and retention (EPR
) effect. After being internalized by cancer cells, under the action of high GSH and esterase, the DAS and CP could be released in situ for inhibiting Src activity and inducing cell apoptosis, respectively, resulting in a synergistic anti-tumor effect
[11][158].
2. Synergistic PDT/PTT-Chemo Therapy
Phototherapy, including PDT and PTT, is a tumor resection and function-preserving interventional therapy, which shows great potential in clinical application. In the process of phototherapy, non-toxic phototherapeutic agents (PSs or PTAs) can be activated upon light irradiation, thus inducing cell death without causing undesirable collateral damage to normal tissue. However, it is difficult to completely eradicate solid tumors with single phototherapy. It has been reported that the combination of PTT and/or PDT with chemotherapy can provide therapeutic advantages including (1) giving play to synergistic effects during treatment. (2) Decreasing the undesirable side effects from anticancer drugs via lowering the drug dosage. (3) Facilitating the deep tumor penetration of chemotherapeutic drugs under hyperthermia treatment (PTT). (4) Promoting the cellular internalization of drugs in the presence of a large amount of ROS (PDT). (5) Inducing an immune response by phototherapy, including innate/adaptive immunity and antitumor immunity, to maximize the therapeutic outcome
[12][13][105,159]. The unfavorable pharmacokinetic properties and desynchrony in the tumor accumulation of chemotherapeutic drugs and phototherapeutic agents still hinder the success of synergistic PDT/PTT-chemotherapy. Therefore, phototherapeutic nanomedicine has also aroused great interest in order to continuously improve its performance
[14][15][97,160], among which conjugated nanomedicine occupies an important position
[16][17][161,162]. As for synergistic PDT-chemotherapy, for example, Chen and co-workers developed a supramolecular system with optimized PS (4,4-difluoro-boradiazaindacene, BODIPY) and anti-cancer drug (PTX) loading efficiency. The adamantyl BODIPY (Ada-BODIPY) and PTX (Ada-PTX) were connected to the block copolymer (PEG-PGA-β-CD) through the host–guest interaction between adamantane and β-cyclodextrins (β-CD), followed by being prepared into nanoparticles of Ada-PTX (60%)-BODIPY(40%)-PNS. These nanoparticles remained in the precise drug loading ratio during circulation with the minimized pre-mature release of drugs. Upon NIR laser irradiation, ROS could be generated efficiently for PDT as well as the cleavage of ROS-sensitive aminoacrylate linker in Ada-PTX. Finally, PDT and cascaded Ada-PTX activation showed a significant inhibitory effect on tumor growth
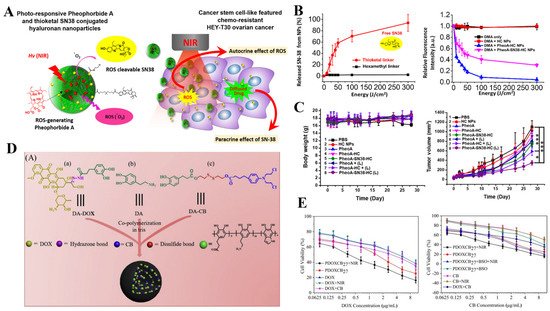
[18][163]. A similar nanosystem (PheoA-SN38-HC) has been developed by Lee and co-workers, which contains the ROS-cleavable thioketal-SN38 for the drug release during PDT, showing good tumor targeting for CD44 positive cancer cells and effective tumor inhibition mediated by synergistic PDT-chemotherapy
[19](Figure 7A–C) [164].
Figure 7. (A) Schematic representation of demonstrating combination of therapeutic hyaluronan nanoparticles conjugated with photodynamic pheophorbide A and ROS-cleavable thioketal-SN38 and drug delivery mechanism of nanoparticles. (B) Characterization of PhoeA-SN38-HC NPs: NIR induced singlet oxygen generation from NPs. In the presence of DMA (100 μM), 1 mg/mL NPs were exposed to light, and fluorescence intensity (λ = 420 nm) of DMA was measured by spectrometer, Light induced drug release from NPs depending on light energy. (C) In vivo PDT treatment with HC-PheoA-SN38: body weights and tumor growth curves of HEY-T30 xenograft BALB/C nude mouse, * p < 0.05 and *** p < 0.001. Reprinted with permission from [164]. Copyright@ Elsevier. (D) The preparation of dual drugs-conjugated PDOXCBs nanoparticles. (E) Both PDOXCB18 and PDOXCB27 cytotoxicity against cancer cells. Reprinted with permission from Ref. [167]. 2021, Elsevier.
Hypoxia is a key feature of the solid tumor microenvironment (TME) resulting from rapid malignant cell proliferation and vascular deformation during tumor angiogenesis, which is not beneficial to PDT. To overcome this problem, Xu and co-workers designed a nanoplatform self-assembled from amphiphilic oligomer Ce6-PEG Platinum(IV) (Ce6-PEG-Pt(IV), CPP) with upconversion nanoparticles (UCNPs) in the hydrophobic core. In this system, platinum(IV) diazido complexes bearing cis-diamine ligands can be activated to produce O
2 as well as cytotoxic Pt(II) simultaneously upon laser illumination, successfully compensating for the consumption of O
2 during the PDT process. The released active platinum(II) could also trigger efficient chemotherapeutic effects, resulting in dramatically enhanced synergistic PDT-chemotherapy
[21][165].
As for synergistic PTT-chemotherapy, Li and co-workers designed a prodrug–hemicyanine conjugate (Cy-azo) based nanoplatform to achieve the combination of H-aggregation-improved PTT and sequential hypoxia-activated chemotherapy. In Cy-azo, nitrogen mustard was introduced into the NIR fluorescent group heptamethyl cyanamide through an azo bond, and the superposition of the conjugates promoted H aggregates, showing higher photothermal conversion efficiency than traditional cyanine dyes. In addition, under hypoxic conditions, the nitrogen mustard can be activated due to the cleavage of azo bonds and released in the hypoxic TME to induce cell death, thereby greatly reducing the side effects of chemotherapy
[22][166]. In another work, Zhou and co-workers prepared the dual drugs-conjugated polydopamine nanoparticles (PDOXCBs) through the one-pot aqueous copolymerization of two dopamine prodrugs, which combined the NIR-mediated PTT with cocktail chemotherapy into one nanoplatform. Upon NIR irradiation, PDOXCBs presented a dramatic photothermal effect with the assistance of polydopamine nanoparticles as PTAs. Meanwhile, chemotherapeutic drugs, including DOX and chlorambucil (CB), could be released from the nanoplatforms under the stimuli of pH 5.0 and the reduced environment, respectively. The synergistic PTT-chemotherapy based on PDOXCB27 upon NIR irradiation displayed a highly lowered IC
50 value on MCF-7 cells and a combination index of 0.36, revealing a promising combination between PTT and cocktail chemotherapy
[20](Figure 7D,E) [167].
3. Synergistic Immune-Chemo Therapy
In recent years, with the development of molecular biology and tumor biology, tumor immunotherapy has become a new treatment method with a good application prospect. During cancer immunotherapy, the collective immune system can be activated by strengthening the natural immune defense of patients to fight against cancer cells and relieving the immunosuppressive microenvironment, to eradicate tumors and inhibit tumor metastasis and recovery. On the one hand, immunotherapy is aimed at training immune cells to recognize and remove target cells carrying tumor antigens, and enhancing immune-mediated tumor cell lysis, displaying the advantages of good curative effect, fewer adverse reactions and the prevention of recurrence. On the other hand, the down-regulation of the immunosuppressive signal pathways in tumor tissues could also facilitate the final immunotherapeutic effects
[23][168]. So far, there have been several types of immunotherapy achieving great success in tumor therapy, such as immune checkpoint blockade (ICB), adoptive T cell transfer, cytokine therapy, agonist immunotherapy, vaccines and so on. However, due to the complexity and heterogeneity of tumors, systemic defects, such as immune escape and immunotoxicity of tumors make the overall efficacy of immunotherapy only about 20%. It needs to further improve the efficiency of tumor immunotherapy via inhibiting immune escape and enhancing the immunotherapeutic response rate. Among the various therapeutic strategies to improve the efficacy of immunotherapy, drug conjugates have been recognized as one of the good choices. Nano-drug delivery systems can enhance the retention, accumulation, penetration and target cell uptake of tumor immunotherapeutic drugs in tumor sites
[24][25][169,170].
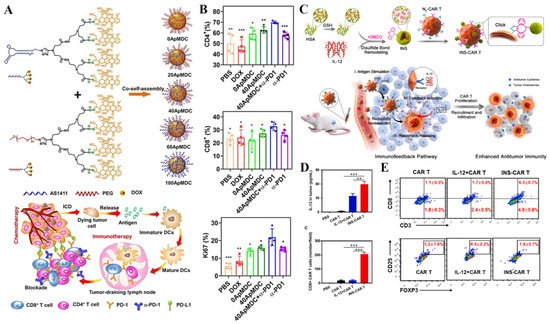
Besides, the combination of immunotherapy with other therapeutic modalities, such as chemotherapy and phototherapy, increases the immunotherapeutic effects. It has been recognized that chemotherapy can induce immunogenic cell death (ICD) to express or release damage-associated molecular patterns (DAMPs), including calreticulin (CRT), high-mobility group box 1 (HMGB-1), and adenosine triphosphate (ATP). These DAMPs are capable of enhancing the immunogenicity of cancer cells and stimulating the immune system to fight against tumors. Given this, Geng and co-workers developed the aptamer-drug conjugate nanomicelles to facilitate the antitumor immune response via DOX-mediated chemotherapy. In detail, an amphiphilic telodendrimer (ApMDC) consisting of an aptamer AS1411 and a monodendron connected with four DOX through acid-labile hydrazone spacers was firstly synthesized, followed by co-self-assembly with an ApMDC analog, in which the aptamer is substituted by PEG. Based on their results, the optimized micelles could induce ICD. Besides, the chemotherapy also promoted the tumor-specific immune responses of anti-PD-1 therapy
[26](Figure 8A,B) [171]. In another work, Hu and co-workers developed a ROS-sensitive nanosystem (denoted as pep-PAPM@PTX) for synergistic chemotherapy and ICB therapy. The PD-L1-targeting D-peptide (NYSKPTDRQYHF, pep) was conjugated to the carrier materials and exposed to the surface of micelles with the function of anti-PD-L1 therapy. Accordingly, this micelle could bind to PD-L1 on the cell surface and promote its uptake via the lysosome-involved internalization, thus inhibiting PTX-activated PD-L1 upregulation and downregulating PD-L1 expression. It dramatically facilitated T cell infiltration and enhanced tumor immune activation, finally synergizing with PTX-mediated chemotherapy to achieve promising anticancer effects against triple-negative breast cancer (TNBC)
[27][172]. Bai and co-workers designed a GSH/pH dual-responsive prodrug nano-platform (known as DDA) for synergistic chemotherapy/PDT/immunotherapy. The nano-platform can effectively enhance the immune response by promoting the maturation of dendritic cells and reducing the number of immunosuppressive immune cells, showing the enhanced adjuvant effect of anti-PD-1 therapy
[28][173].
Figure 8. (A) Preparation of ApMDC and their self-assembled nanomicelles with tunable surface density of aptamers, initiation of antitumor immune responses of checkpoint blockade therapy by tumor-targeting, yet enhanced, chemotherapy. (B) In vivo antitumor immune responses: quantitative analysis of tumor infiltrating CD4+, CD8+, and level of Ki67 in the tumor-draining lymphoid node after treatment with PBS, free DOX, 0ApMDC, 40ApMDC, 40ApMDC + a-PD1 and a-PD1, respectively, * p < 0.05, ** p < 0.01 and *** p < 0.001. Reprinted with permission from Ref. [171]. 2021, Wiley-VCH GmbH. (C) Schematic illustration of IL-12 nanostimulant-engineered CAR T cells (INS-CAR T) biohybrids with immunofeedback to enhance immunotherapy in solid tumors. The CAR T cell-mediated INS delivery system elicited CAR T cell infiltration and enhanced immune responses. (D) IL-12 accumulation in tumor of NOD/SCID mice at 48 h post last administration and the average CAR T cell number in each field (600 μm × 600 μm) of tumor tissues was calculated from 50 fields, ** p < 0.01 and *** p < 0.001. (E) Representative flow cytometry analysis of the ratios of the percentages of CD8+ CAR T cells and coexpression of CD25 and Foxp3 among CD4+ CAR T cells in tumor tissues. Reprinted with permission from Ref. [176]. 2022, Elsevier.
In addition to be applied for synergistic immune-chemo therapy, conjugated nanomedicine serves as a promising tool, and can also enhance the therapeutic efficacy of immunotherapy alone or other forms of synergistic immunotherapy, which is necessary to be discussed in this section. The company named Cyrtlmmune Sciences has developed a conjugated nanomedicine-related antitumor drug CYT-6091 (trade name: Aurimune
TM) for cytokine immunotherapy. As mentioned in the previous section, gold nanoparticles can serve as PTAs and drug carriers simultaneously. The CYT-6091 was synthesized by covalent binding with recombinant human tumor necrosis factor (rhTNF) onto colloidal gold nanoparticles coated with mercapto functionalized PEG. It can be specifically stored in tumor tissues and has no obvious toxic and side effects
[30][174]. It also provided the potential to combine with gold nanoparticles-mediated thermal therapy.
More recently, Xue and co-workers reported that the CD73 enzyme was highly expressed in tumor cells and immunosuppressive cells, including regulatory T cells (T
reg cells), myeloid suppressor cells (MDSCs) and M2-like tumor-associated macrophages (TAM.M2). However, CD73 was negative for non-immunosuppressive cells, known as lytic T lymphocytes, natural killer cells (NK cells) and dendritic cells (DC cells). Based on these findings, they developed an IR-700 dye-coupled anti-CD73 antibody (α-CD73-Dye), which could bind to CD73
+ cells selectively. Upon NIR laser irradiation, these conjugates could perform photoimmunotherapy against targeted cells and prevent tumors from acquiring resistance to ICB, finally leading to advanced tumor eradication
[31][175].
Another effective cancer immunotherapeutic modality is chimeric antigen receptor T-cell immunotherapy (CAR-T therapy), which is an adoptive T cell metastasis therapy (adaptive T cell metastasis, ACT), which infuses the patient’s T cells back into the patient to fight cancer. Compared with ordinary T cells, CAR-T is not restricted by the major histocompatibility complex (MHC), thus avoiding the immune escape of tumor cells with the low expression of MHC molecules on their surface. However, the immunosuppressive tumor microenvironment inhibits the infiltration of T cells, limiting the effect of CAR-T therapy. Luo and co-workers combined human serum albumin (€) and IL-12 into nanoparticles, which were further modified onto the surface of CAR-T cells via bioorthogonal chemistry to yield IL-12 nonstimulant engineering CAR T cells (INS-CAR T) hybrids. The IL-12 released from nanoplatforms can promote the secretion of CCL
5, CCL
2 and CXCL
10, thus increasing the infiltration of CD8+ CAR T cells, relieving the immunosuppressive TME. Based on their results, the anti-tumor ability of CAR-T cells has been improved and the growth of solid tumors was inhibited with negligible side effects
[29](Figure 8C–E) [176].
4. Synergistic PTT-TDT
As the prominent character of solid tumors, hypoxia impedes the therapeutic effect of oxygen-dependent radical-based cancer therapy, such as PDT and radiotherapy. To address these hypoxia issues, researchers have developed another type of oxygen-independent radical-based cancer treatment strategy, known as TDT. During TDT, the alkyl radicals can be produced upon heating with high efficiency due to the presence of thermally decomposable radical initiators, such serving as radical donors. More importantly, the aforementioned PTT could also serve as a heat source to induce the generation of alkyl radicals. Upon NIR light irradiation, light-triggered heat and heat-caused alkyl radicals can jointly damage vital cellular components and further induce cell death
[32][33][177,178]. To improve the accumulation of radical initiators and PTAs in tumors and avoid the undesired pre-mature release of them during blood circulation, the PTA-initiator conjugated nanomedicine has also been developed for enhanced synergistic PTT-TDT. Xia and co-workers conjugated the photothermal PSs (porphyrin) with radical initiator 2,2′-azobis [2-(2-imidazolinI-2-yl) propane dihydrochloride (AIBI) and prepared the nanoparticles (tripolyphosphate (TPP)-NN NPs) with the assistance of pluronic F-127 as surfactant. The aggregated porphyrin could generate heat upon 638 nm laser illumination and then trigger initiator AIBI to produce alkyl radicals to induce cell death even in a hypoxia environment. TPP-NN NPs have shown the potential to inhibit the growth of cervical tumors without notable systemic toxicity
[34][179].
In
the our
esearchers' previous study, an all-organic nanoparticle (denoted as ZPA@HA-ACVA-AZ NBs) realized the “precise strike” of hypoxic tumors via synergistic PTT/TDT. The loading strategy of radical initiators (ACVA) was optimized by the conjugation of alkyl chain-functionalized initiators ACVA-HDA to HA, thus averting the unfavorable adverse effect in normal tissues while improving the efficiency for the targeted delivery of radical initiators to solid tumors. Then, this amphiphilic hyaluronic acid (HA)-based lipoid (HA-ACVA-AZ) was used as a carrier to encapsulate the special zinc(II) phthalocyanine aggregates (ZPA), acting as PTAs for highly efficient PTT upon 808 nm laser irradiation. Therefore, the sequentially generated heat and alkyl radicals could simultaneously trigger cell death and restrain cancer metastasis under the action of PTT/TDT and CA IX inhibition
[35][180].
The Our
esearchers' group also developed carrier-free nano-theranostic agents (denoted as AIBME@IR780-APM NPS) for magnetic resonance imaging (MRI)-guided synergistic PTT/TDT. As an extension of previous work,
the researchers we were devoted to the incorporation of diagnostic functions into the nanoplatform to improve the accuracy of synergistic therapy. As for the subject of this nanomedicine, the first IR780 derivative, IR780-ATU, was designed to conjugate the chelating agents (acylthiourea, ATU) with PTA with the purpose of chelating transition metal Mn
2+ ions to perform the T1-weighted contrast-enhanced MRI. The other derivative, IR780-PEG, renders a nanosystem with high sterical stability, and increased solubility of hydrophobic IR780/dimethyl 2,2′-azobis(2-methylpropionate) (AIBME, radical initiator) and reduced risk from reticuloendothelial system (RES) uptake. Upon IR780-mediated PTT launched under NIR laser irradiation, AIBME could generate highly cytotoxic alkyl radicals, combing the heat from PTT to synergistically induce cell death, ignoring tumor hypoxia
[36][181].