Japanese pharmaceutical cosmetics, often referred to as quasi-drugs, contain skin-lightening active ingredients formulated to prevent sun-induced pigment spots and freckles. Their mechanisms of action include suppressing melanin production in melanocytes and promoting epidermal growth to eliminate melanin more rapidly. For example, arbutin and rucinol are representative skin-lightening active ingredients that inhibit melanin production, and disodium adenosine monophosphate and dexpanthenol are skin-lightening active ingredients that inhibit melanin accumulation in the epidermis. In contrast, oral administration of vitamin C and tranexamic acid in pharmaceutical products can lighten freckles and melasma, and these products are more effective than quasi-drugs. On the basis of their clinical effectiveness, skin-lightening active ingredients can be divided into four categories according to their effectiveness and adverse effects.
- skin-lightening
- pharmaceutical cosmetics
- quasi-drug
- ingredient
- melasma: pigment spots
1. Development of Skin-Lightening Active Ingredients
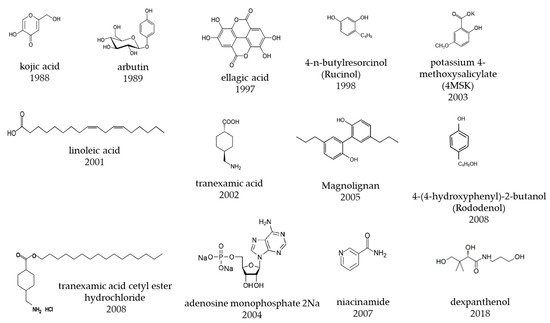
| Approved Year | Generic Name | Development Company | Chemical Name/Substance Name | Main Mechanism of Action |
|---|---|---|---|---|
| placenta extract | ||||
| 1983 | magnesium ascorbyl phosphate (APM) | Takeda Pharmaceutical Co., Ltd. | magnesium L-ascorbyl-2-phosphate | tyrosinase inhibition |
| 10% Magnesium Ascorbyl Phosphate Formulation | 7% Arbutin Formulation | 0.5% Ellagic Acid Formulation | 1% Kojic Acid and 0.1% Oil-Soluble Licorice Extract Formulation | 2% 4-Hydroxyanisole and 0.01% Vitamin A Acid Formulation | |||
|---|---|---|---|---|---|---|---|
| Test design | Open study | Open study | Open study | Open study | Double-blind controlled study | ||
| Number of cases | 17 | ||||||
| Effectiveness Indices | Skin-Lightening Ingredients | Test Concentration (%) | General Purpose or Japanese Cosmetics Company | Scientific Articles Providing Evidence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Effectiveness of same concentration of cosmetic formulations on human pigment spots has been published in scientific journals and is highly recommended. | tranexamic acid | 2 | General purpose | [12] | [66] | |||||||
| 16 | 13 | ||||||||||||
| arbutin | 3 | General purpose | [13] | [31]18 | 420 | 421 | 1988 | kojic acid | Sansho Seiyaku Co., Ltd. | kojic acid | |||
| Sex | tyrosinase inhibition | ||||||||||||
| – | – | Women | |||||||||||
| 3-O-ethyl ascorbic acid (vitamin C ethyl) | 1 | General purpose | [ | 5 men, 13 women | 14] | Men and women | [8] | 1989 | arbutin | Shiseido Co., Ltd. | hydroquinone-β-D-glucopyranoside | tyrosinase inhibition | |
| Age | – | – | Unknown | 28–50 years old (average 39 years old) | 34–85 years old (average 62.6 years old) | ||||||||
| magnesium L-ascorbyl-2-phosphate (APM) | 3 | General purpose | [15] | [2] | 1994 | ascorbyl glucoside (AA-2G) | Hayashibara Co., Ltd., Kaminomoto Co., Ltd., Shiseido Co., Ltd. | L- ascorbic acid 2-O-α-glucoside | tyrosinase inhibition | ||||
| 1997 | |||||||||||||
| Location | – | – | Unknown | Face | Forearm | Face | |||||||
| ellagic acid | 0.5 | General purposeellagic acid | Lion Corporation | ellagic acid | |||||||||
| [ | 9 | ] | [ | Period | tyrosinase inhibition | ||||||||
| 36 | ] | – | 3 months to 1 year | 1 to 3 months | 8 and 16 weeks | 24 weeks | Observation up to 48 weeks after 24 weeks of application | ||||||
| kojic acid | 2.5, 0.5 | General purpose | [16][17] | [23 | 1998 | Rucinol | ® | Kurarey Co., Ltd. POLA Chemical industries, Inc. |
4-n-butylresorcinol | tyrosinase inhibition | |||
| , | 24 | ] | Effectiveness judgment | Skin color value (color difference meter) | Visual observation | Visual observation | Close-up photograph determination and skin color value (image analysis) | Visual observation | Visual observation | ||||
| linoleic acid | 0.1 | General purpose | [18] | [52] | 1999 | Chamomile ET | Kao Corporation | Matricaria chamomilla | L Extract | ||||
| Effectiveness ratio | endothelin blocker | ||||||||||||
| Effective (improved or much improved) or higher | 58.80% | 3 months 0%, | 6 months 15.4%, 1 year 66.7% |
30.80% | 8 weeks 5.6%, 16 weeks 22.2% |
52.60% | |||||||
| 4-n-butyl resorcinol, | 0.3 | 56.30% | General purpose | [19] | [ | 2001 | linoleic acid S | Sunstar Inc. | linoleic acid | tyrosinase degradation, stimulation of epidermal turn over | |||
| 37 | ] | Slightly effective (slightly improved) or more | 88.20% | 3 months 81.2%, 6 months 100%, 1 year 100% |
69.20% | 8 weeks 66.7%, 16 weeks 77.8% |
79.30% | ||||||
| chamomile extract | 0.5 | Kao Corporation | 84.10% | [20] | [51] | 2002 | tranexamic acid (t-AMCHA) |
Shiseido Co., Ltd. | trans-4-aminocyclohexane carboxylic acid | inhibition of prostaglandin E | 2 | production by anti-plasmin | |
| Adverse effects | – | None | None | Irritation in a few cases, but no serious adverse effects | Redness 56%, burning 34%, desquamation 24%, itching 16%, irritation 7%, decoloration 9%. |
– | |||||||
| adenosine monophosphate | 3 | Otsuka Pharmaceutical Co., Ltd. | 2003 | 4MSK | |||||||||
| [ | 21 | ] | [75] | References | [7] | [Shiseido Co., Ltd. | 4] | [8] | [87potassium 4-methoxysalicylate | ] | [9] | [36]tyrosinase inhibition | |
| [ | 10 | ] | [ | 26] | [6] | [86] | [6] | [86] | |||||
| B | Effectiveness of higher concentrations than those used in cosmetics on human pigment spots has been published in scientific journals and is recommended. | oil-soluble licorice extract containing 50% glabridin | 0.2 | General purpose | [22] | [ | 2004 | Vitamin C ethyl | Nippon Hypox Laboratories, Inc. | 3-O-ethyl ascorbic acid | tyrosinase inhibition | ||
| 89 | ] | ||||||||||||
| niacinamide | 5, 4 | General purpose | [23][24] | [77,78] | 2004 | Energy signal AMP | ® | Otsuka Pharmaceutical Co., Ltd. | |||||
| placenta extract | 3 | General purpose | adenosine mono phosphate | [ | stimulation of epidermal turnover | ||||||||
| 25 | ] | [ | 17] | 2005 | Magnolignan | ® | Kanebo Cosmetics Inc. | 5,5-dipropyl-biphenyl-2,2-diol | inhibition of tyrosinase maturation, cytotoxicity to melanocytes | ||||
| retinol | 0.15 | General purpose | [26] | [90 | 2007 | D-Melano (niacinamide W) | P&G Maxfactor | niacinamide | suppression of melanosome transfer | ||||
| 2008 | Rhododenol | ® | Kanebo Cosmetics Inc. | 4-(4-hydroxyphenyl)-2-butanol, Rhododendrol | tyrosinase inhibition, cytotoxicity of melanocytes | ||||||||
| 2008 | TXC | CHANEL | tranexamic acid cetyl ester hydrochloride | inhibition of prostaglandin E | 2 | production | |||||||
| 2009 | ascorbyl tetraisopalmitate | Nikko Chemicals Co., Ltd. | ascorbyl tetra-2-hexyldecanoate | tyrosinase inhibition | |||||||||
| 2018 | dexpanthenol W (PCE–DP) | POLA ORBIS Holdings Inc. | dexpanthenol | enhance energy production of epidermal cells | |||||||||
2. Report on the Effectiveness of a Formulation Containing a Lightening Agent and a Spot Remedy for Senile Pigmented Lesions
3. Effectiveness Indices of Lightening Ingredients Developed in Japan
| ] | ||||||
| ascorbic acid 2-O-α-glucoside (AA-2G) | ||||||
| 20 (iontophoresis) | ||||||
| General purpose | ||||||
| [ | ||||||
| 27 | ||||||
| ] | ||||||
| [ | ||||||
| 91 | ||||||
| ] | ||||||
| azelaic acid. | 20 | General purpose | [28] | [92] | ||
| C | No effectiveness for human pigment spots has been published in scientific journals and may be considered, but evidence is insufficient | potassium 4-methoxysalicylate | 1, 3 | Shiseido Co. Ltd. | ||
| dexpanthenol | POLA ORBIS HOLDINGS INC. | |||||
| D | Not recommended, because of toxicity data published in scientific journals | Rhododenol | 2 | Kanebo Cosmetics Inc. | [29] | [46] |
| Magnolignan | 0.5 | Kanebo Cosmetics Inc. | [29] | [46] | ||
| ascorbyl tetra-2-hexyldecanoate | Nikko Chemicals Co. Ltd. | [30][31][32] | [14,15,16] |
