Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tushar Garg and Version 3 by Beatrix Zheng.
Image-guided locoregional therapies play a crucial role in the management of patients with hepatocellular carcinoma (HCC). Transarterial therapies consist of a group of catheter-based treatments where embolic agents are delivered directly into the tumor via their supplying arteries. Some of the transarterial therapies available include bland embolization (TAE), transarterial chemoembolization (TACE), drug-eluting beads–transarterial chemoembolization (DEB–TACE), selective internal radioembolization therapy (SIRT), and hepatic artery infusion (HAI).
- hepatocellular carcinoma
- transarterial chemoembolization
- hepatic artery infusion
- selective internal radioembolization therapy
- bland embolization
1. Initial Angiography
Either the common femoral artery (CFA) or radial artery (RA) access can be used for performing transarterial embolization [1][89]. A 4–6 Fr vascular sheath is used with either approach. If the RA access is selected, a cocktail of heparin and one or more vasodilators (nitroglycerin, verapamil, or nicardipine) is generally administered intraarterially via the sheath once access has been achieved [2][90].
Mesenteric angiography is performed to visualize and define the relevant vascular anatomy and identify tumoral arterial supply, as well as collaterals and arterial supply at risk of non-target embolization [3][91]. A superior mesenteric angiogram is obtained first to exclude aberrant vascular anatomy and confirm portal vein patency on delayed imaging. Then a celiac arteriogram is performed to identify target (hepatic artery branches) and non-target arteries and hypervascular tumors [4][92]. In situations where, extrahepatic arterial recruitment is suspected, extrahepatic vessels (phrenic, gastric, and internal mammary) are also evaluated [5][93]. Frequently, intraprocedural cone-beam CT technology is utilized to identify and/or confirm arterial supply of targeted tumors [6][94].
Once the procedure is completed, all the catheters and sheaths are removed, and hemostasis is achieved by either manual compression or with the help of a closure device [7][95].
2. Embolization Techniques
2.1. Bland Embolization (TAE)
In TAE, after following the steps described in the previous section, lobar embolization or subselective embolization can be performed depending on the arterial distribution, location of the tumor, and tumor burden. TAE is usually performed by using embolic agents ranging from 40 to 120 µm in size that may be spherical or non-spherical, resorbable or non-resorbable, calibrated or non-calibrated [8][9][96,97]. Particles are mixed with iodinated contrast, normal saline solution, and an antibiotic in some cases. Embolization is performed to the endpoint of stasis of flow in the target vessel(s).
2.2. Transarterial Chemoembolization (TACE)
In TACE, following the above steps, a microcatheter is used to subselectively catheterize target branches. The goal of subselective catheterization is to maximize the delivery of chemoembolic material to tumor, while minimizing, as much as possible, delivery to the normal liver. Once the catheter position is optimized, conventional or DEB–TACE can be performed under real-time fluoroscopic observation. For conventional TACE, the prescribed chemotherapy is combined with ethiodized oil and contrast and then continuously administered until the peritumoral venous plexus is visualized. However, this approach might be difficult to achieve in big tumors. For DEB–TACE, the endpoint of administration is sub-stasis of flow within the supplying vessel. Caution must be used to prevent over-embolization, resulting in reflux into non-target liver and non-target organs (i.e., bowel, pancreas, and spleen). In cTACE, particles or gelfoam are often delivered after chemoembolic material to prevent “washout” of chemotherapy. If multiple tumors are present, the best approach is to target each individual tumor as selectively as possible. Classically, only one lobe (right or left) is treated in a single setting; however, this approach is variable with the ability to perform subselective targeted delivery of chemoembolic material. If lipiodol or radiodense beads are utilized, non-contrast cone-beam CT or non-contrast CT can be obtained post-delivery to visualize the extent of lipiodol deposition. Figure 13A–D shows CT and MRI of a patient who underwent cTACE. Figure 24A–F shows the CT and angiograms of a patient who underwent DEB–TACE.
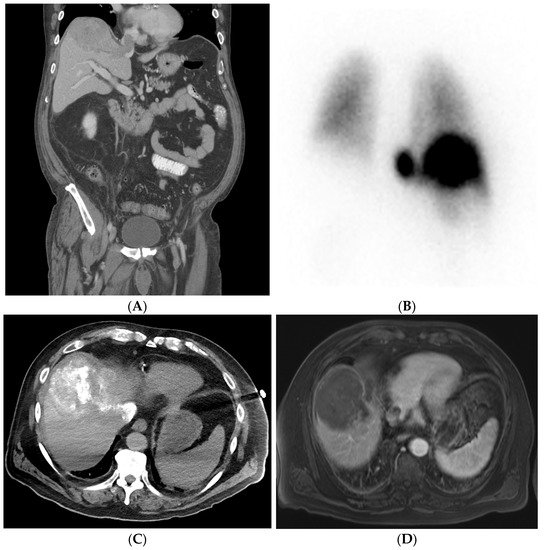
Figure 13. Patient with right lobe hepatocellular carcinoma (HCC) with extension into the hepatic vein causing thrombosis. Pre-procedural investigation showed 30% shunt fraction, and therefore the patient was treated with cTACE. (A) Pre-procedural coronal CT shows right lobe HCC lesion with hepatic vein thrombosis. (B) A 99mTc-MAA SPECT/CT shows a lung shunt with a lung shunt fraction of 30%. (C) Intraoperative CT showing good lipiodol uptake in the treated lesion areas. (D) Follow-up MRI showing reduced non-enhancing HCC and hepatic vein thrombus due to good response after treatment.
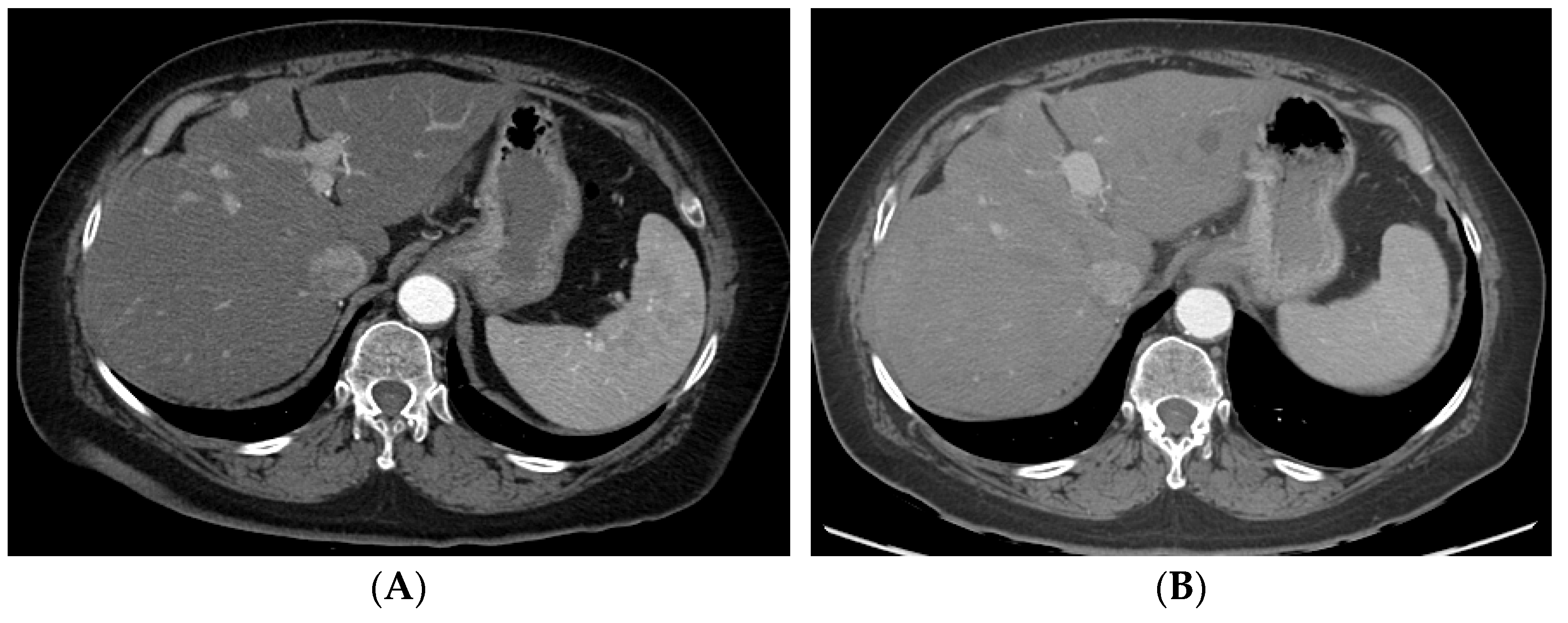
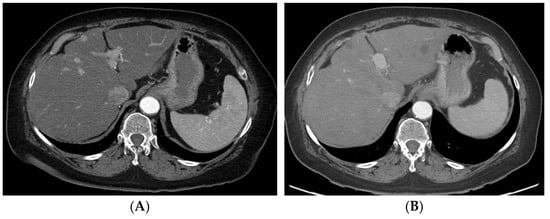
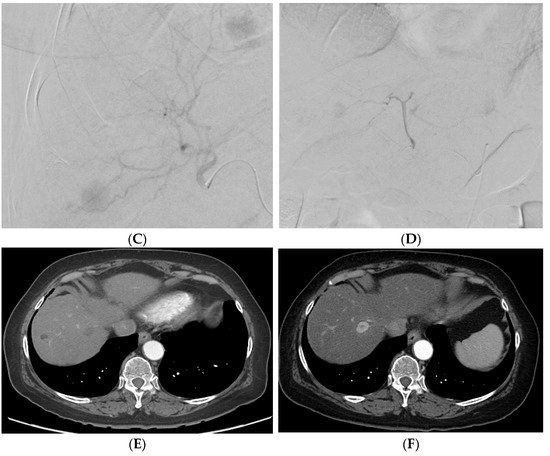
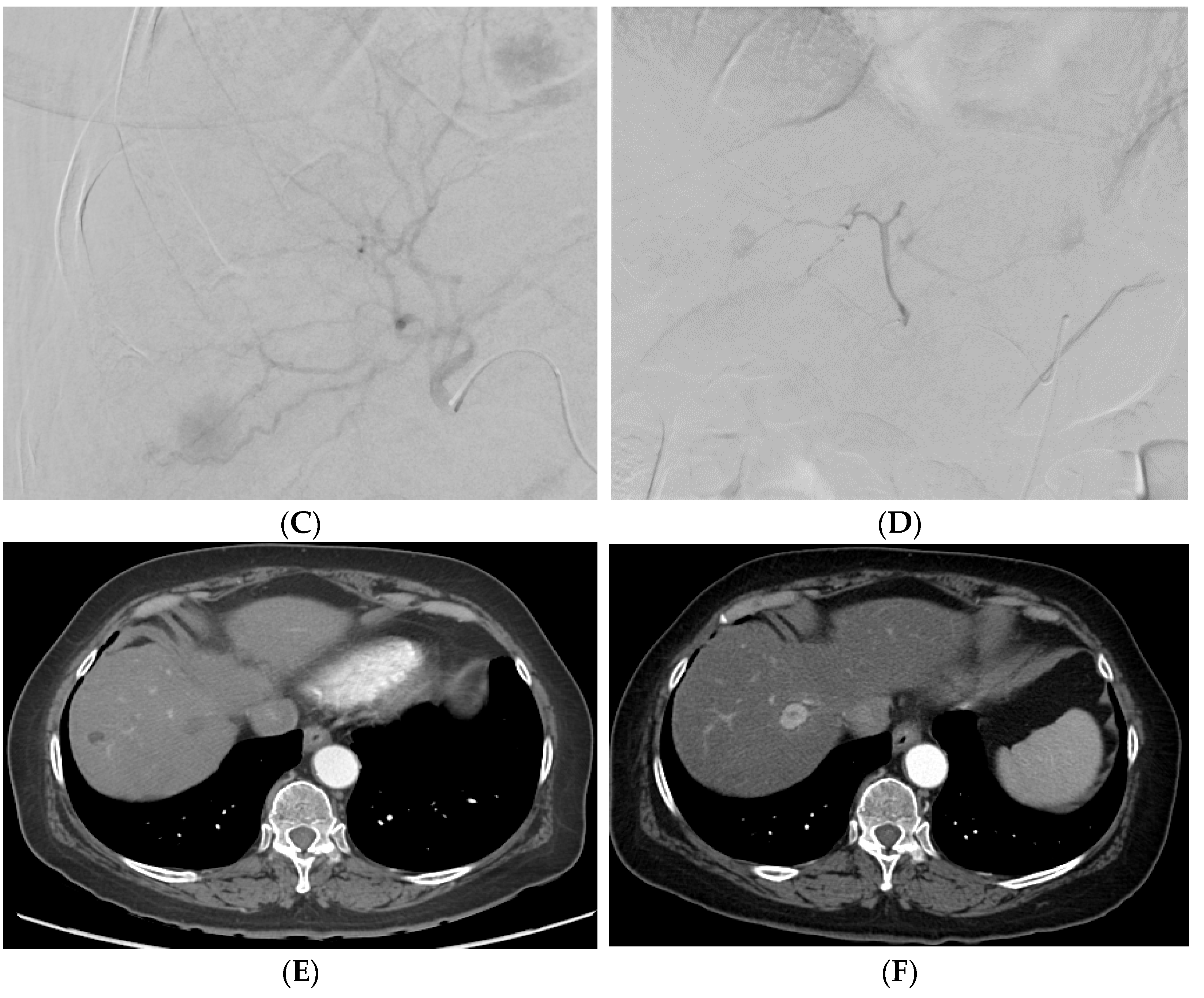
Figure 24. A patient with multifocal HCC who underwent DEB–TACE for its management. CT showing multifocal HCC involving the right (A) and left lobe (B). Common hepatic artery angiogram showing tumor blush (C), which completely disappeared after DEB–TACE (D). Follow-up CT showing good tumor response in the right (E) and left (F) lobe of the liver.
2.3. Selective Internal Radiation Therapy (SIRT)
With SIRT/Y-90-radioembolization, the added step of technetium-99 m macroaggregated albumin (Tc-99 m MAA) shunt researchtudy, or “mapping”, prior to the SIRT is necessary. Following diagnostic angiography and subselective catheterization of supplying tumoral feeders, approximately 4 mCi of Tc-99 m MAA is delivered into the lobe or segment containing the target tumor. The target artery may be identified with the aid of cone-beam CT. After the injection of Tc-99 m MAA, the patient is taken for planar nuclear imaging or SPECT/CT in order to assess for any shunting (specifically to the lung) and assess technical success by visualizing Tc-99 m MAA deposition within targeted tumors. The patient then returns for Y-90 delivery 1–4 weeks following the shunt researchstudy (although “same-day” mapping and delivery are also performed at select centers). The prescribed dose of Y-90 depends on multiple factors such as tumor size and number, liver function, and the microparticle of choice. Y-90-radioembolization is performed from the same catheter position utilized for the Tc-99m MAA shunt researchstudy. SPECT/CT is then again sometimes obtained post-procedure to assess Y-90 distribution and dose delivery to the targeted tumor. Figure 35A–D shows the CT, SPECT, and angiogram of a patient who underwent segment-two segmentectomy.
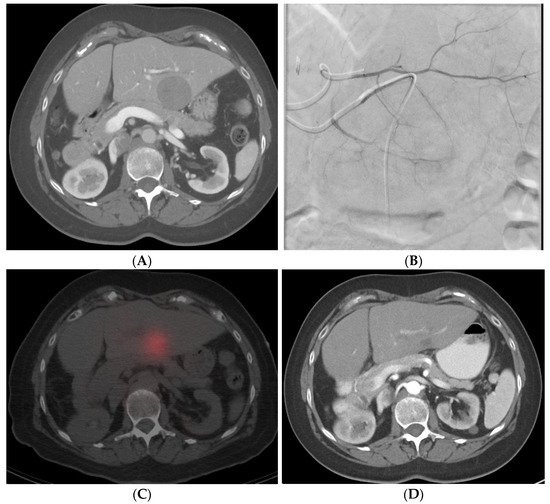
Figure 35. Radiation segmentectomy in a patient with HCC with a past surgical history of right hepatectomy. (A) CT showing a large lesion in the left lobe of the liver. (B) Left hepatic artery angiogram showing vessels supplying the tumor. (C) SPECT-CT imaging after left segment-2 sub-segmentectomy, showing good dose delivery. (D) Follow-up CT showing good response in the treated area.
2.4. Hepatic Artery Infusion (HAI)
In hepatic artery infusion (HAI), chemotherapy is delivered to the tumor by infusing it into the hepatic artery by placing either a surgically implanted pump/port or by placing a percutaneous catheter, which can be connected to an external pump [10][11][12][98,99,100]. For percutaneously placed catheters, the Seldinger technique is used to access the artery and place infusion catheters. Left subclavian and right femoral artery accesses are frequently used. The most commonly used site for access is the common femoral artery, as it is easier to access due to its superficial and less torturous course. In the “fixed-catheter-tip” technique, the distal tip of the catheter is fixed to the gastroduodenal artery, and the chemotherapy is infused to the tumor with the help of a side hole which is positioned into the proper hepatic artery [13][101]. Another technique which can be used is the “long tapered catheter placement” technique [14][102]. In this technique, the catheter is positioned distally into the segmental hepatic artery, and the side hole of the catheter is placed at the origin of the proper hepatic artery [15][103].
