Polymers are being used in many applications all around the world. However, there are some drawbacks in the properties of polymers that could hamper their usage in certain applications. Therefore, a new material polymer composite was introduced. A polymer composite is a polymer-based material with the addition of a filler. Many researchers have reported the improvement in the properties of a polymer when a filler was introduced. This helps minimize the disadvantages of using a polymer. Fillers are materials added to resins or binders (polymer/concrete/metal/ceramic) to improve their specific properties as they are turned into a new form of material called a “composite.” Dolomite is a type of sedimentary carbonate rock that consists mainly of dolomite mineral. The use of dolomite in a polymer composite system has gained increasing attention in recent years after researchers successfully proved that it is capable of improving mechanical and physical properties of various polymeric materials.
- dolomite
- polymer composite
- mechanical properties
- chemical modification
- hybrid filler
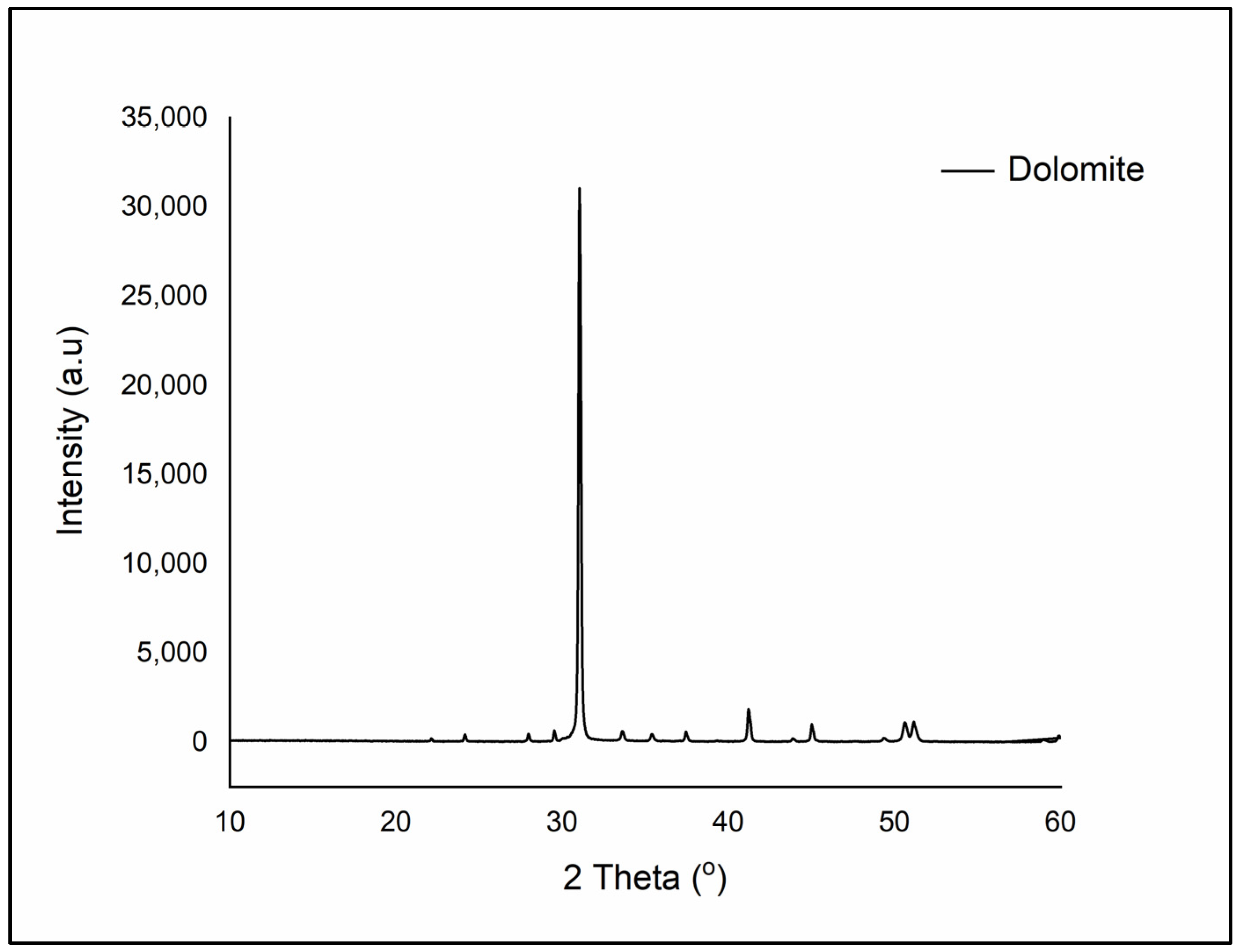
1. Dolomite



| Physical Properties | Dolomite | [15] | Calcite | [16] | |||
|---|---|---|---|---|---|---|---|
| Chemical composition | (CaMg(CO | 3 | ) | 2 | ) | CaCO | 3 |
| Color | Colorless, white, pink, gray, brown, black | White, colorless, gray, red, green, blue, yellow, orange, brown | |||||
| Streak | White | White | |||||
| Cleavage | Perfect, rhombohedral, three directions | Perfect, rhombohedral, three directions | |||||
| Mohs hardness | 3.5–4 | 3 | |||||
| Specific gravity | 2.8–2.9 | 2.7 | |||||
| Diagnostic properties | Rhombohedral cleavage | Rhombohedral cleavage | |||||
| Crystal system | Hexagonal | Trigonal |

| Polymer Matrix | Primary Filler | Secondary Filler | Processing Method | Filler Loading | Tensile | Elongation at Break (%) | Impact Strength | Hardness | Flexural | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strength (MPa) | Modulus | Stress (MPa) | Strength (MPa) | Modulus (MPa) | |||||||||
| To adjust magnesium concentration in soil | [19] | ||||||||||||
| Epoxy resin | Carbon nanotubes (CNTs) | Dolomite | Mechanical stirrer | Chemical vapor deposition | [37] | ||||||||
| Aquaculture | To reduce phosphorus | [21] | |||||||||||
| Ceramics | |||||||||||||
| 0% | 20.89 | 961.31 (MPa) | N/A | N/A | N/A | N/A | N/A | N/A | As a source of lime | [9] | In kiln control | [17] | |
| 1% | 28.52 | 1152.87 (MPa) | N/A | N/A | N/A | N/A | N/A | N/A | Chemical | To make salts, e.g., magnesia | [22] | ||
| 3% | 33.83 | 1188.1 (MPa) | N/A | N/A | N/A | N/A | N/A | N/A | Construction | In cement and concrete manufacturing | [17][20][23] | As flooring material | [ |
| 5% | 3][24] | As road construction material | [24][25] | ||||||||||
| 4.93 | 1216.5 (MPa) | N/A | N/A | N/A | N/A | N/A | N/A | Plastic for structural application | As a filler | [4] | |||
| Physically hybrid | Paint | As a filler | [26] | As an aid in pigmentation | [27 | ||||||||
| 0% | ] | ||||||||||||
| 20.89 | 961.31 (MPa) | N/A | N/A | N/A | N/A | N/A | N/A | Wastewater treatment | For copper ion adsorption | [5] | |||
| 1% | 26.71 | 1139 (MPa) | N/A | N/A | N/A | N/A | N/A | N/A | Membrane application | As low-cost membranes or substrates | [28] | ||
| 3% | 29.97 | 1159.8 (MPa) | N/A | N/A | N/A | N/A | Plastics | As a filler to improve the mechanical properties | [29][30] | ||||
| Pharmaceutical | As a supplementary source of calcium and magnesium | [31] | As an osmotic oral laxative | [31] | |||||||||
2. Dolomite as a Hybrid Filler in a Polymer Composite
| N/A | |||||||||||||||
| N/A | |||||||||||||||
| 5% | |||||||||||||||
| 27.94 | |||||||||||||||
| 1148.7 (MPa) | N/A | N/A | N/A | N/A | N/A | N/A | |||||||||
| Epoxy resin | Glass fiber | Dolomite | Hand layup | 0% | 59.8 | N/A | N/A | N/A | 2.4 (J) | 102.12 (HRL) | 72.3 | N/A | [38] | ||
| 5% | 52 | N/A | N/A | N/A | 2.7 (J) | 107.27 (HRL) | 62.8 | N/A | |||||||
| 10% | 45.9 | N/A | N/A | N/A | 3.4 (J) | 109.60 (HRL) | 54.3 | N/A | |||||||
| 15% | 42.8 | N/A | N/A | N/A | 3.8 (J) | 112.26 (HRL) | 48..8 | N/A | |||||||
| Epoxy resin | Glass fiber | Dolomite | Hand layup | 0% | 282.58 ± 12.96 | 2.32 ± 0.14 (GPa) | N/A | N/A | 6.1 ± 0.37 (J) | 101.32 ± 3.08 (HV) | 285.3 ± 13.12 | N/A | [39] | ||
| 5% | 268.59 ± 9.43 | 2.94 ± 0.l5 (GPa) | N/A | N/A | 7.2 ± 0.36 (J) | 103.76 ± 2.89 (HV) | 273 ± 9.65 | N/A | |||||||
| 10% | 244.69 ± 7.80 | 3.26 ± 0.13 (GPa) | N/A | N/A | 8.8 ± 0.35 (J) | 105.64 ± 3.23 (HV) | 262.8 ± 7.12 | N/A | |||||||
| 15% | 227.6 ± 7.10 | 2.44 ± 0.10 (GPa) | N/A | N/A | 10.2 ± 0.41 (J) | 108.22 ± 2.3 (HV) | 249.9 ± 7.24 | N/A | |||||||
| VTRM | 0% | 374.46 ± 17.80 | 3.54 ± 0.21 (GPa) | N/A | N/A | 8.1 ± 0.46 (J) | 102.94 ± 3.18 (HV) | 337.4 ± 14.25 | N/A | ||||||
| 5% | 337.33 ± 10.80 | 4.15 ± 0.17 (GPa) | N/A | N/A | 9.2 ± 0.37 (J) | 106.3 ± 225 (HV) | 331.8 ± 10.12 | N/A | |||||||
| 10% | 293.05 ± 11.65 | 4.33 ± 0.22 (GPa) | N/A | N/A | 10.6 ± 0.48 (J) | 109.06 ± 3.08 (HV) | 327.6 ± 12.38 | N/A | |||||||
| 15% | 270.43 ± 7.12 | 4.02 ± 0.12 (GPa) | N/A | N/A | 13.4 ± 0.39 (J) | 112.08 ± 2.38 (HV) | 319.9 ± 7.60 | N/A | |||||||
| Low-density polyethylene (LDPE) | Kenaf core fiber (KCF) | Dolomite | Internal mixer | 0% | N/A | 109.37 (MPa) | 1.47 | N/A | 3.52 (kJ/m | 2 | ) | N/A | N/A | N/A | [40] |
| 3% | N/A | 116.72 (MPa) | 1.50 | N/A | 3.73 (kJ/m | 2 | ) | N/A | N/A | N/A | |||||
| 6% | N/A | 122.52 (MPa) | 1.53 | N/A | 3.82 (kJ/m | 2 | ) | N/A | N/A | N/A | |||||
| 9% | N/A | 123.48 (MPa) | 1.60 | N/A | 4.03 (kJ/m | 2 | ) | N/A | N/A | N/A | |||||
| 12% | N/A | 124.50 (MPa) | 1.81 | N/A | 4.11 (kJ/m | 2 | ) | N/A | N/A | N/A | |||||
| 15% | N/A | 131.30 (MPa) | 1.86 | N/A | 4.69 (kJ/m | 2 | ) | N/A | N/A | N/A | |||||
| 18% | N/A | 143.46 (MPa) | 1.91 | N/A | 5.29 (kJ/m | 2 | ) | N/A | N/A | N/A | |||||
| Phenolic | Dolomite | Multiwalled carbon nanotubes (MWCNTs) | Ball milling machine | 0% | N/A | N/A | N/A | N/A | N/A | 25(H | R | ) | N/A | N/A | [41] |
| 1% | N/A | N/A | N/A | N/A | N/A | 36(H | R | ) | N/A | N/A | |||||
| 3% | N/A | N/A | N/A | N/A | N/A | 45(H | R | ) | N/A | N/A | |||||
| 5% | N/A | N/A | N/A | N/A | N/A | 52(H | R | ) | N/A | N/A | |||||
| Phenolic | Dolomite | Carbon nanotubes (CNTs) | Mechanical stirrer | Chemical vapor deposition | [42] | ||||||||||
| 0 | N/A | N/A | N/A | N/A | N/A | 25 | N/A | N/A | |||||||
| 1 | N/A | N/A | N/A | N/A | N/A | 39 | N/A | N/A | |||||||
| 3 | N/A | N/A | N/A | N/A | N/A | 46 | N/A | N/A | |||||||
| 5 | N/A | N/A | N/A | N/A | N/A | 50 | N/A | N/A | |||||||
| Physically hybrid | |||||||||||||||
| 0 | N/A | N/A | N/A | N/A | N/A | 25 | N/A | N/A | |||||||
| 1 | N/A | N/A | N/A | N/A | N/A | 30 | N/A | N/A | |||||||
| 3 | N/A | N/A | N/A | N/A | N/A | 37 | N/A | N/A | |||||||
| 5 | N/A | N/A | N/A | N/A | N/A | 38 | N/A | N/A | |||||||
| Waterborne polyurethane (WPU) | Fibrous palygorskite (PAL) | Dolomite | Mechanical stirring | 0% | 4.9 | N/A | N/A | 9.4 | N/A | N/A | N/A | N/A | [10] | ||
| WPU/PAL10% | 3.5 | N/A | N/A | 8.8 | N/A | N/A | N/A | N/A | |||||||
| WPU/DOL 10% | 4. | N/A | N/A | 9.5 | N/A | N/A | N/A | N/A | |||||||
| WPU/MIX 10% | 8. | N/A | N/A | 8.1 | N/A | N/A | N/A | N/A | |||||||
| Polypropylene (PP) | Wood flour | Dolomite | High-intensity mixer and single-screw extruder | 0% | 27.1 | 474 (MPa) | N/A | 29.4 | N/A | N/A | 37.1 | 1013 | [43] | ||
| 3% | 24.8 | 615 (MPa) | N/A | 20.1 | N/A | N/A | 42.4 | 1592 | |||||||
| 6% | 24.4 | 620 (MPa) | N/A | 22.2 | N/A | N/A | 41.0 | 1617 | |||||||
| 9% | 22.8 | 627 (MPa) | N/A | 19.4 | N/A | N/A | 41.8 | 1673 |
References
- Mehmood, M. Dolomite and Dolomitization Model—A Short Review. Int. J. Hydrol. 2018, 2, 549–553.
- Banerjee, A. Estimation of Dolomite Formation: Dolomite Precipitation and Dolomitization. J. Geol. Soc. India 2016, 87, 561–572.
- Hussin, K.; Jamaludin, S.; Mohd, C.; Ghazali, R.; Idris, S.; Salleh, N.; Nizar, K. The Development of Artificial Marble from Dolomite (Batu Reput) in Perlis. KUKUM Eng. Res. Semin. 2006, 101–106.
- Adesakin, A.O.; Ajayi, O.O.; Imosili, P.E.; Attahdaniel, B.E.; Olusunle, S.O.O. Characterization and Evaluation of Mechanical Properties of Dolomite as Filler in Polyester. Chem. Mater. Res. 2013, 3, 36–40.
- Cao, Z.; Chen, P.; Yang, F.; Wang, S.; Zhong, H. Transforming Structure of Dolomite to Enhance Its Ion-Exchange Capacity for Copper (II). Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 201–208.
- Nik Nur Azza, N.A.; Ong, H.L.; Noorina Hidayu, J.; Akil, H.M.; Sam, S.T. Analysis of Ground Dolomite: Effect of Grinding Time on the Production of Submicron Particles. Appl. Mech. Mater. 2014, 679, 145–148.
- Ridhwan, J.N.M.; Noimam, N.Z.; Mohd Salleh, M.A.A.; Sam, S.T.; Musa, L.; Nik Yahya, N.Z. The Effect of Different Sizes “Batu Reput” (Dolomite) as a Filler in SMR L and ENR-50. Adv. Mater. Res. 2013, 795, 383–387.
- Salleh, M.N.; Kasim, F.H.; Ismail, K.N.; Ghazali, C.M.R.; Saad, S.A.; DAud, S. Characterization and Application of Dolomite Rock in Perlis. In Proceedings of the 1st International Conference on Natural Resources Engineering & Technology 2006, Putrajaya, Malaysia, 24–25 July 2006; pp. 465–470.
- Tengku Mustafa, T.N.A.S.; Munusamy, S.R.R.; Uy Lan, D.N.; Yunos, N.F.M. Physical and Structural Transformations of Perlis Carbonate Rocks via Mechanical Activation Route. Procedia Chem. 2016, 19, 673–680.
- Ni, L.; Mao, Y.; Liu, Y.; Cai, P.; Jiang, X.; Gao, X.; Cheng, X.; Chen, J. Synergistic Reinforcement of Waterborne Polyurethane Films Using Palygorskite and Dolomite as Micro/Nano-Fillers. J. Polym. Res. 2020, 27, 23.
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, Nucleation and Growth of Dolomite in the Laboratory and Sedimentary Environment: A Review. Sedimentology 2015, 62, 1749–1769.
- Abdul Samad, H.; Abd Rashid, R. Influence of Dolomite and Granite Waste Content on the Properties of Artificial Marble. IOP Conf. Ser. Mater. Sci. Eng. 2020, 713, 012017.
- Perlis Dolomite Industries. Available online: https://www.perlisdolomite.com.my/?playlist=30d262f&video=2f0c00c (accessed on 26 June 2022).
- Iqbal, Y.; Leu, L.-C.; Fahad, M.; Ubic, R. Characterization of Mineral Ores from Northern and Northwest Pakistan. JOM 2012, 65, 73–79.
- Dolomite Mineral Physical—Optical Properties, Occurrence and Uses. Available online: https://geologyscience.com/minerals/dolomite/ (accessed on 26 June 2022).
- Calcite Mineral|Uses and Properties. Available online: https://geology.com/minerals/calcite.shtml (accessed on 26 June 2022).
- Abdalqader, A.; Sonebi, M. Dolomitic Filler in Self-Compacting Concrete: A Review. RILEM Tech. Lett. 2020, 5, 75–84.
- Murtaja, Y.; Lapčík, L.; Sepetcioglu, H.; Vlček, J.; Lapčíková, B.; Ovsík, M.; Staněk, M. Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior. Nanotechnol. Rev. 2022, 11, 312–320.
- Karaca, S.; Gurses, A.; Ejder, M.; Acikyildiz, M. Adsorptive Removal of Phosphate from Aqueous Solutions Using Raw and Calcinated Dolomite. J. Hazard. Mater. 2006, 128, 273–279.
- Ye, H.; Fu, C.; Yang, G. Influence of Dolomite on the Properties and Microstructure of Alkali-Activated Slag with and without Pulverized Fly Ash. Cem. Concr. Compos. 2019, 103, 224–232.
- Kavitha, M.; Linga, D.; Adhikari, S.; Anikuttan, K.; Prabu, D. Effect of Lime, Dolomite and Gypsum on Phosphorus Reduction Potential in Freshwater. Int. J. Appl. Pure Sci. Agric. 2016, 2, 44–50.
- Wonyen, D.G.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.C. A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite). Minerals 2018, 8, 354.
- John, V.M.; Damineli, B.L.; Quattrone, M.; Pileggi, R.G. Fillers in Cementitious Materials—Experience, Recent Advances and Future Potential. Cem. Concr. Res. 2018, 114, 65–78.
- Hamizah, A.S.; Rashita, A.R.; Malek, S. Characterization and Evaluation of Dolomite and Kaolin as Filler on the Properties of Poly Art Marble. Mater. Today Proc. 2020, 29, 173–178.
- Haritonovs, V.; Tihonovs, J.; Smirnovs, J. High Modulus Asphalt Concrete with Dolomite Aggregates. Transp. Res. Procedia 2016, 14, 3485–3492.
- Solihin; Sulistiyono, E. Synthesis of Magnesium Carbonate Using Indonesian Dolomite. Adv. Mater. Res. 2015, 1112, 546–549.
- Youssef, E.A.M. Characterization, surface modification, and evaluation of Egyptian dolomite ore as an extender pigment for paint. Pigment. Resin Technol. 2002, 31, 226–233.
- Bessa, L.P.; Terra, N.M.; Cardoso, V.L.; Reis, M.H.M. Macro-Porous Dolomite Hollow Fibers Sintered at Different Temperatures toward Widened Applications. Ceram. Int. 2017, 43, 16283–16291.
- Osman, A.F.; Siah, L.; Alrashdi, A.A.; Ul-Hamid, A.; Ibrahim, I. Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process. Polymers 2021, 13, 274.
- Lim, K.C.; Osman, A.F.; Ahmad Fauzi, A.A.; Alrashdi, A.A.; Abdul Halim, K.A. The Mechanical and Thermal Properties of Poly(Ethylene-Co-Vinyl Acetate) (PECoVA) Composites with Pristine Dolomite and Organophilic Microcrystalline Dolomite (OMCD). Polymers 2021, 13, 3034.
- Mohamed El-Mezayen, A.; Mohamed Saleh, G.; Mohamed Abdou El-Desoky, H.; Mohd Said Khalil, B.; Samy, A.M. Mineralogical and Chemical Studies on Some Minerals Used in Pharmaceuticl Industries in Egypt. Al-Azhar J. Pharm. Sci. 2014, 50, 181–190.
- Saba, N.; Tahir, P.; Jawaid, M. A Review on Potentiality of Nano Filler/Natural Fiber Filled Polymer Hybrid Composites. Polymers 2014, 6, 2247–2273.
- Przekop, R.E.; Jakubowska, P.; Sztorch, B.; Kozera, R.; Dydek, K.; Jałbrzykowski, M.; Osiecki, T.; Marciniak, P.; Martyła, A.; Kloziński, A.; et al. Opoka—Sediment Rock as New Type of Hybrid Mineral Filler for Polymer Composites. AppliedChem 2021, 1, 90–110.
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review of the Mechanical and Thermal Properties of Graphene and Its Hybrid Polymer Nanocomposites for Structural Applications. J. Mater. Sci. 2018, 54, 5992–6026.
- Matykiewicz, D.; Barczewski, M.; Mousa, M.S.; Sanjay, M.R.; Siengchin, S. Impact Strength of Hybrid Epoxy–Basalt Composites Modified with Mineral and Natural Fillers. ChemEngineering 2021, 5, 56.
- Jaafar, M. Development of Hybrid Fillers/Polymer Nanocomposites for Electronic Applications. In Hybrid Nanomaterials: Advances in Energy, Environment and Polymer Nanocomposites; Kumar Srivastava, S., Mittal, V., Eds.; Scrivener Publishing: Beverly, MA, USA, 2017; pp. 349–369.
- Saleh, S.S.M.; Wei, Y.S.; Mohammad, N.F.; Abdullah, S.F.A.; Akil, H.M.; Saliu, H.R. Tensile Properties of CNTs-Dolomite Hybrid Filled Epoxy Composites. In Proceedings of the AIP Conference Proceedings, Ariyalur, India, 29 June 2021; AIP Publishing LLC: Melville, NY, USA, 2021.
- Verma, S.K.; Gupta, A.; Singh, T.; Gangil, B.; Jánosi, E.; Fekete, G. Influence of Dolomite on Mechanical, Physical and Erosive Wear Properties of Natural-Synthetic Fiber Reinforced Epoxy Composites. Mater. Res. Express 2019, 6, 125704.
- Verma, S.K.; Gangil, B.; Gupta, A.; Rajput, N.S.; Singh, T. Dolomite Dust Filled Glass Fiber Reinforced Epoxy Composite: Influence of Fabrication Techniques on Physicomechanical and Erosion Wear Properties. Polym. Compos. 2021, 43, 551–565.
- Shamsuri, A.A.; Mohd Zolkepli, M.N.; Mohamed Ariff, A.H.; Sudari, A.K.; Abu Zarin, M. A Preliminary Investigation on Processing, Mechanical and Thermal Properties of Polyethylene/Kenaf Biocomposites with Dolomite Added as Secondary Filler. J. Compos. 2015, 2015, 760909.
- Saleh, S.S.M.; Akil, H.M.; Abdul Kudus, M.H.; Zakaria, M.R. A comparative Study of Dolomite and MWCNTS-Dolomite as Fillers in Phenolic Composites. Malays. Polym. J. 2014, 9, 67–69.
- Saleh, S.S.M.; Akil, H.M.; Abdul Kudus, M.H.; Ahmad, K.R.; Ahmad Bakhtiar, N.S.A. Effect of Different Hybrid Method on Properties of Carbon Nanotubes/Dolomite Hybrid Filled Phenolic Composites. Procedia Chem. 2016, 19, 45–49.
- Özdemir, E.; Ayrilmis, N.; Mengeloglu, F. Effect of Dolomite Powder on Combustion and Technological Properties of WPC and Neat Polyproppylene. J. Chil. Chem. Soc. 2017, 62, 3716–3720.
- Gangil, B.; Ranakoti, L.; Verma, S.K.; Singh, T. Utilization of Waste Dolomite Dust in Carbon Fiber Reinforced Vinylester Composites. J. Mater. Res. Technol. 2022, 18, 3291–3301.
- Amiri, R.-S.N.; Tirri, T.; Wilen, C.-E. Flame Retardant Polyurethane Nanocomposite: Study of Clay Dispersion and Its Synergistic Effect with Dolomite. J. Appl. Polym. Sci. 2012, 129, 1678–1685.
- Vijayaraghavan, J.; Jeevakkumar, R.; Venkatesan, G.; Rengasamy, M.; Thivya, J. Influence of Kaolin and Dolomite as Filler on Bond Strength of Polyurethane Coated Reinforcement Concrete. Constr. Build. Mater. 2022, 325, 126675.
