Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by niki papapostolou and Version 2 by Catherine Yang.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder characterized by intense pruritus, eczematous lesions, and relapsing course. It presents with great clinical heterogeneity, while underlying pathogenetic mechanisms involve a complex interplay between a dysfunctional skin barrier, immune dysregulation, microbiome dysbiosis, genetic and environmental factors. All these interactions are shaping the landscape of AD endotypes and phenotypes. In the “era of allergy epidemic”, the role of food allergy (FA) in the prevention and management of AD is a recently explored area.
- atopic dermatitis
- food allergy
- food sensitization
1. Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting more than 230 million people worldwide [1][2][1,2]. With an increasing global prevalence, up to 20% of children and 10% of adults in high-income countries suffer from AD [3][4][5][3,4,5], while AD’s morbidity burden designates the disease as the skin disorder with the highest impact on quality of life in terms of disability-adjusted years [6].
AD presents with great heterogeneity in terms of severity, clinical features, and course, with a complex underlying pathophysiology [5], which involves an interplay between the dysfunctional skin barrier, immune dysregulation—starting with a core T helper 2 (TH2) response accompanied by IgE sensitization to environmental allergens and progressing with a widening of the adaptive immunity with TH1, TH17, and TH22 responses—and skin microbiome dysbiosis [7][8][7,8]. In addition, genetic susceptibility, along with filaggrin gene’s mutations being the central but not the only recognized genetic disorder, and environmental factors such as ultraviolet radiation, air pollution, water hardness, household hygiene, and climate change contribute to the multidimensional model of atopic dermatitis [9].
As allergic diseases have reached epidemic proportions, with the first epidemic wave of respiratory allergy increasing about 50 years ago, we are now riding a second epidemic wave of “food allergy” [10][11][10,11]. In respect to the TH2 endotype, atopic dermatitis has been proposed as the first “step” in a long although debatable pathway known as the “atopic march”, with food allergy, allergic rhinitis, and asthma presenting either concomitantly or at a later stage [12].
Food allergy (FA) is defined as a food hypersensitivity reaction mediated by immunologic mechanisms, while the term IgE-mediated food allergy is used when the role of IgE is underlined [13]. Food sensitization refers to the production of food-allergen-specific IgE but is not synonymous with food allergy, as individuals can produce specific IgEs to foods without presenting symptoms upon exposure. Hence, sensitization is prerequisite but not synonymous with food allergy [14]. Food allergen sensitization can be identified by skin prick testing or in vitro immunoassays for specific IgE to whole-allergens extracts or allergens components (pure allergen proteins) [15][16][15,16]. Not only IgE-mediated food allergy but also other endotypes of food allergy (either non-IgE-mediated or mixed) have been implicated either in the exacerbations or in the morbidity of AD complexing even more the landscape of atopic dermatitis–food allergy interaction [17][18][17,18] (Figure 1).
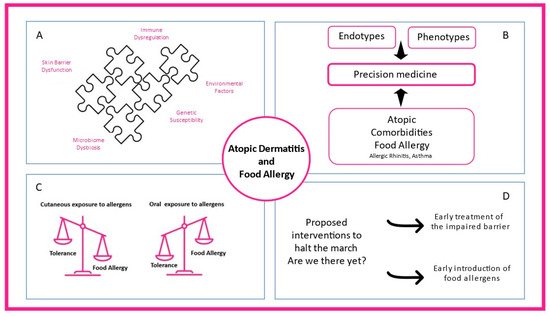
Figure 1. The complex interplay between atopic dermatitis and food allergy. (A) Skin barrier dysfunction, microbiome dysbiosis, immune dysregulation, environmental factors, and genetic susceptibility contribute to atopic dermatitis heterogeneity. (B) Different endotypes, phenotypes, and atopic comorbidities present in clinical practice shaping precision medicine interventions. (C) While oral exposure to food allergens through the gastrointestinal tract promotes food tolerance, cutaneous allergen exposure can lead to food allergy. (D) Early treatment of the impaired skin barrier and early introduction of food-specific food allergens in high-risk infants are proposed mechanisms to prevent atopic dermatitis and food allergy.
2. Association between Food Sensitization and Food Allergy with Atopic Dermatitis
2.1. Transepidermal Water Loss and Skin Barrier Impairment
A close relationship between atopic dermatitis, food sensitization, and food allergy, especially in childhood, is well-established. Epidermal barrier dysfunction and transepidermal water loss (TEWL) constitute a cornerstone in atopic dermatitis pathophysiology and precedes both the occurrence of atopic dermatitis and food allergy [19][20][21][21,35,36]. Kelleher et al. found that increased TEWL at two days after birth was associated with increased risk of development of AD at one year of age [20][35]. A constantly growing body of evidence supports that food allergy follows atopic dermatitis diagnosis and leads to the reasonable conclusion that AD is involved in the causal pathway towards food allergy [19][22][23][21,23,37]. Although less commonly, food allergy might precede AD onset in a small number of children [24][38]. In the Isle of Wight cohort, filaggrin loss-of-function mutations were associated with food sensitization in the early years and food allergy later in childhood, suggesting that skin barrier function per se is important in the development and persistence of food allergy [25][39].
2.2. Food Sensitization and Food Allergy among Patients with AD
Numerous studies support that more severe phenotypes of AD are associated with more frequent diagnosis of food allergy, ranging between 33% to 39%, with occasional studies reporting higher rates up to 80% [26][27][28][29][40,41,42,43], while food allergy prevalence in the general population is estimated about 0.1–6% [30][44]. Hence, atopic dermatitis is proposed as a major risk factor for food sensitization and IgE-mediated food allergy [22][31][23,45]. Population-based studies have shown that the likelihood of food sensitization is up to six times higher at 3 months of age in patients with AD compared to healthy controls [22][23]. When including patients with established AD, the prevalence of food sensitization is up to 66%, while proven food allergy by oral food challenges is up to 81% [22][23]. The Danish Allergy Research cohort (DARC) showed that up to 53% of children with AD, aged 6 months to 6 years, were sensitized to food allergens, while food allergy was confirmed in 15% of them [19][21]. In the Health Nut study, a large population-based Australian study (n = 4453), infants with AD were 6 times more likely to have egg allergy (95% CI 4.6, 7.4) and 11 times more likely to have peanut allergy (95% CI 6.6, 18.6) by 12 months than infants without AD at 12 months of age [32][46]. Although previous studies suggested that the presence of AD, particularly in its more severe form, is associated with a prolonged course of milk and egg allergy [33][34][47,48], recent data were not confirmatory [35][49]. Such discrepancies might be attributed to different study designs and populations with different disease severity.
2.3. Conclusions
Although AD is proposed as a major risk factor for food sensitization and food allergy, its occurrence depends on both severity and chronicity of AD. Rates of food sensitization are high in patients with AD, but frequency of IgE-mediated food allergy confirmed by oral food challenges varies, with more severe AD linked more frequently with food allergy.
