Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Bo Zhang and Version 2 by Peter Tang.
The CXCL12/CXCR4 biological axis is a coupled molecular pair, which is formed by the interaction of CXCL12 and its corresponding receptor CXCR4, and is closely related to intercellular messaging and cell migration.
- CXCL12/CXCR4 axis
- chemokines
- cancer therapy
1. Basic Concepts
Chemokine CXCL12 is a steady-state chemokine that is also known as stromal cell-derived factor-1 (SDF-1). There are multiple isoforms of CXCL12, including α, β, and γ. CXCL12α is rapidly degraded in the blood by protein hydrolysis, while the β isomer is more resistant to blood-dependent degradation and represents a powerful stimulator of neoangiogenesis. CXCL12 is expressed at high levels in cancerous tissues; the main producer of CXCL12 is tumor-associated fibroblasts [1][7].
CXCR4, also known as CD184, is a functional receptor of CXCL12 and consists of 352 amino acids with seven membrane-splicing structures; this is an evolutionarily highly conserved G protein-coupled receptor (GPCR) [2][8]. Unlike other chemokine receptors, CXCR4 regulates inflammatory and immune processes by primarily acting on leukocytes [3][2]. CXCR4 is expressed in a variety of cell types, including lymphocytes, hematopoietic stem cells, endothelial cells, epithelial cells, stromal fibroblasts, and cancer cells; the expression of CXCR4 is upregulated under conditions of hypoxia, stress, and injury [4][5][6][9,10,11].
The binding of CXCL12 to CXCR4 can release free heterotrimeric G proteins into the cytoplasm that are composed of Gα, Gβ, and Gγ subunits. When the signaling pathway is activated, guanosine diphosphate (GDP) on the trimer is replaced by guanosine triphosphate (GTP); subsequently, the G protein is dissociated into a βγ dimer and an α monomer binding to GTP [7][8][12,13]. In turn, these activate various intracellular signal transduction pathways and downstream effectors that can trigger a range of biological phenomena, including cell survival, proliferation, migration and apoptosis, angiogenesis, actin polymerization, cytoskeletal rearrangement, and extracellular matrix remodeling [9][10][11][12][13][14,15,16,17,18].
2. Downstream Signaling Pathways of the CXCL12/CXCR4 Axis
The specific mechanisms responsible for the actions of the CXCL12/CXCR4 axis are illustrated in Figure 1. Several typical signaling pathways are involved. (i) Activation of the PI3K/AKT pathway mediates cell survival. The GβGγ dimer and Gα monomer can activate phosphoinositol-3 kinase (PI3K), thus leading to the phosphorylation of various signaling factors that can induce cell growth and survival by activating serine/threonine protein kinase AKT [3][2]. In cancer, the PI3K/AKT pathway plays an important role in promoting the epithelial–mesenchymal transition (EMT) and cell metastasis. (ii) Activation of the PLC/IP3 pathway mediates the release of the Ca2+ ion. The Gβγ subunit activates phosphatidylinositol-specific phospholipase C-β (PLC-β), which breaks down phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 can then bind to endoplasmic reticulum-specific receptors and promote the release of Ca2+ [14][19]. DAG and Ca2+ co-activate mitogen-activated protein kinase (MAPK), thus promoting cell migration [15][20]. (iii) Activation of the Ras/ERK pathway mediates gene expression. The Gα monomer activates extracellular signal-regulated kinase (ERK) through the Ras pathway; ERK then enters the nucleus and works with other regulatory proteins to activate cellular transcription factors that synergize with NF-κB to promote gene expression and cell cycle progression [16][21].
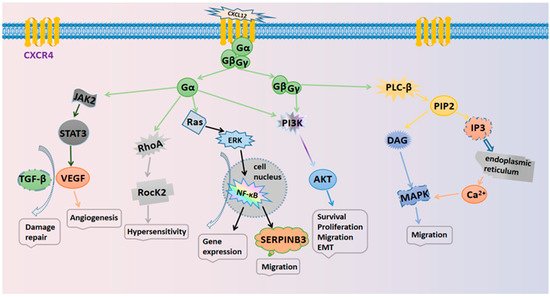
Figure 1.
The signaling pathways in the CXCL12/CXCR4 biological axis.
3. Mediated Physiological Effects in Tumors
The CXCL12/CXCR4 biological axis plays a crucial role in developmental processes in the body. During the early stages of development, CXCL12/CXCR4 signaling is involved in the recruitment of uterine natural killer cells, the migration of progenitor cells, placental formation, embryogenesis, and the development of the central nervous system and cardiovascular organogenesis [17][22]. In adulthood, CXCL12/CXCR4 signaling can influence the migration of stem cells in the bone marrow or ecotone to repair tissue damage [18][23]. In addition, the CXCL12/CXCR4 axis mediates a variety of physiological roles, including cell invasion, hematopoiesis, tissue repair, embryonic development, and immune cell trafficking [19][20][24,25]. The main physiological effects of CXCL12/CXCR4 signaling are given below.
- The regulation of cell growth and proliferation. The CXCL12/CXCR4 axis can induce the proliferation of tumor cells via activation of the ERK and AKT signaling pathways. Tumor cells can express CXCL12 in a paracrine mode; this stimulates tumor stromal cells to produce tumor necrosis factors, thus promoting the growth of tumor cells
- Mediating the repair of tissue damage
- . The CXCL12/CXCR4 axis is crucial for stem cell homing and can recruit stem cells to the site of injury to then differentiate into functional cells to repair tissue damage. In addition, the CXCL12/CXCR4 axis can upregulate the expression of VEGF to mediate the repair of injured tissues together with transforming growth factor (TGF-β) [18].
-
The regulation of cell growth and proliferation. The CXCL12/CXCR4 axis can induce the proliferation of tumor cells via activation of the ERK and AKT signaling pathways. Tumor cells can express CXCL12 in a paracrine mode; this stimulates tumor stromal cells to produce tumor necrosis factors, thus promoting the growth of tumor cells [26
- [21].
- ].
- The regulation of the cell motility and migration
- . The upregulation of CXCR4 on the cell surface allows the cells to efficiently recruit sources of chemokines. For example, CXCL12 can be expressed in the bone marrow (BM) and promote the migration of myeloma cells [
-
The regulation of the cell motility and migration. The upregulation of CXCR4 on the cell surface allows the cells to efficiently recruit sources of chemokines. For example, CXCL12 can be expressed in the bone marrow (BM) and promote the migration of myeloma cells [27]. CXCL12/CXCR4 also enhances cell migration to promote the progression of human ovarian cancer [28
- 22
- ]. CXCL12/CXCR4 also enhances cell migration to promote the progression of human ovarian cancer [23].
- ].
- The mediation of cell adhesion
- . Cell adhesion plays an important role in cell survival, migration, inflammation, angiogenesis, and injury repair. The CXCL12/CXCR4 axis can upregulate the expression of adhesion molecules such as very late antigen-4 (VLA-4) and very late antigen-5 (VLA-5), thereby increasing cell adhesion. VLA-4 increases the adhesion to fibronectin, thus resulting in an increase in overall cell adhesion. In addition, CXCL12 can promote the expression of adhesion molecules
- [24].
- The mediation of cell adhesion. Cell adhesion plays an important role in cell survival, migration, inflammation, angiogenesis, and injury repair. The CXCL12/CXCR4 axis can upregulate the expression of adhesion molecules such as very late antigen-4 (VLA-4) and very late antigen-5 (VLA-5), thereby increasing cell adhesion. VLA-4 increases the adhesion to fibronectin, thus resulting in an increase in overall cell adhesion. In addition, CXCL12 can promote the expression of adhesion molecules [29].
- Participation in angiogenesis
- . Angiogenesis plays a critical role in normal development and the progression of cancer and is closely related to the CXCL12/CXCR4 axis. Activation of the CXCL12/CXCR4 axis prevents the degradation of β-catenin in the cytoplasm and the accumulation of β-catenin in the nucleus, which can induce angiogenesis via the Wnt/β-catenin, MAPK/ERK, and PI3K/AKT pathways
- [
- 25]. The CXCL12/CXCR4 axis can also induce vascular endothelial growth factor (VEGF) expression through the JAK2/STAT3 pathway, inducing tumor angiogenesis [21].
- Participation in angiogenesis
- . Angiogenesis plays a critical role in normal development and the progression of cancer and is closely related to the CXCL12/CXCR4 axis. Activation of the CXCL12/CXCR4 axis prevents the degradation of β-catenin in the cytoplasm and the accumulation of β-catenin in the nucleus, which can induce angiogenesis via the Wnt/β-catenin, MAPK/ERK, and PI3K/AKT pathways [30]. The CXCL12/CXCR4 axis can also induce vascular endothelial growth factor (VEGF) expression through the JAK2/STAT3 pathway, inducing tumor angiogenesis [26].
-
Mediating the repair of tissue damage. The CXCL12/CXCR4 axis is crucial for stem cell homing and can recruit stem cells to the site of injury to then differentiate into functional cells to repair tissue damage. In addition, the CXCL12/CXCR4 axis can upregulate the expression of VEGF to mediate the repair of injured tissues together with transforming growth factor (TGF-β) [23].
4. The CXCL12/CXCR4 Axis and Cancer
In cancer, the CXCL12/CXCR4 biological axis can stimulate the proliferation and metastasis of tumor cells, regulate the inflammatory state of tumors, and activate the immune response within tumors by both autocrine and paracrine factors [26][31]. CXCR4 is expressed at high levels in many solid tumors and hematological tumors and represents an effective target for the diagnosis and treatment of tumors.
4.1. Tumor Inflammation
Inflammation is one of the most important features of tumors [27][33]. Chronic inflammation can initiate and contribute to the development of tumors. In tumor tissue, certain inflammatory factors, such as mononuclear inflammatory cells (MICs) and myeloid-derived suppressor cells (MDSCs), can cause immunosuppression and thus promote tumor development [28][34]. Meanwhile, neutrophils, as the predominant leukocytes, often show contradictory therapeutic effects on cancers. Neutrophils could be polarized into different tumor-associated neutrophils (TAN) in the TME, for which N1-type TAN possesses the antitumor effects while N2-type TAN displays the pro-tumor effects [29][30][35,36].
Inflammation may promote tumor development via the CXCR4 pathway. In the TME, the inflammatory factor tumor necrosis factor α (TNF-α) activates NF-κB, which then enters the nucleus to upregulate the expression of CXCR4, thus promoting metastasis in human neuroblastoma [31][37]. On the surface of human tongue squamous cell carcinoma (TSCC) cells, the interaction of the pro-inflammatory cytokine IL-1β with IL-1 receptor 1 induces the activation of ERK and Notch signaling, both of which promote the expression of CXCR4 receptors, thus promoting tumor growth and metastasis [32][38]. In another study, Zhang et al. reported that miR-302b, a gene that can inhibit CXCR4, efficiently downregulated cancer-related inflammation (CRI) in patients with esophageal cancer (EC) [33][39].
4.2. Tumor Immunity
The CXCL12/CXCR4 axis plays a key role in innate and adaptive immune responses, mediates the retention of hematopoietic stem cells in the bone marrow, and is responsible for the transport of T-cell precursors to the thymus and the clearance of neutrophils from the bone marrow. The CXCL12/CXCR4 axis mediates the metastasis and homing of immune cells at tumor sites, thus affecting the specific immune response. In the TME, high expression levels of CXCR4 increase the infiltration of myeloid-derived suppressor cells (MDSCs) but reduces CD8+ T cell infiltration, thus inhibiting the immune response [34][40]. CXCR4 also increases Treg infiltration in tumors and releases an immunosuppressor of T cells by suppressing IFN-γ production, promoting proliferation, and inhibiting apoptosis [35][41].
In colorectal cancer cells, the CXCL12/CXCR4 axis regulates PTEN to induce M2 polarization by activating the PI3K/Akt signaling pathway. M2-polarized macrophages promote cancer metastasis by promoting EMT and the secretion of VEGF [36][42]. Using an animal model of ovarian cancer, Righi et al. found that the CXCR4 antagonist AMD3100 reduced the infiltration of regulatory T cells (Treg) and significantly enhanced the T cell-mediated anti-tumor immune response [37][43]. In another animal-based study, Yang et al. reported that CXCR4 inhibition on myeloid cells upregulated the secretion of cytokine IL18 and inhibited the Fas/FasL signaling pathway; this promoted the neutrophil-dependent activation of NK cells, thus enhancing anti-tumor immunity and inhibiting tumor growth [38][44].
4.3. Tumor Cells
The CXCL12/CXCR4 axis can interact with multiple cellular signaling pathways via both local autocrine and paracrine mechanisms. In this context, the CXCL12/CXCR4 axis plays a decisive role in the development and progression of cancer, including cell proliferation and metastasis, tumor angiogenesis, the epithelial mesenchymal transition, intracellular calcium increase, and gene transcription [39][40][41][45,46,47].
CXCR4 signaling can prolong the lifespan of stem cells and increase the potential for DNA damage and mutations, thus transforming these cells into cancer stem cells (CSCs), ultimately promoting the progression of cancer [18][23]. The breast tumor leader cells (K14+) can utilize both chemical and mechanical cues by CXCR4 signaling, including low oxygen, collagen density, chemokine gradient, and interstitial fluid flow, in order to guide collective migration [42][48]. In gastric cancer cells, CXCL12/CXCR4 signaling can activate NF-kB; this upregulates the expression of serine protease inhibitor branch B member 3 (SERPINB3), thus promoting the metastasis of tumor cells [43][49]. In bone cancer, CXCR4 mediates pain hypersensitivity by activating the downstream RhoA/ROCK2 pathway [44][50]. In pancreatic ductal adenocarcinoma (PDAC), the CXCL12/CXCR4 axis mediates the desmoplastic reaction; this changes the tumor mechanical microenvironment and promotes drug resistance [45][51]. In addition, the CXCL12/CXCR4 biological axis plays an important role in the progression of several cancers, including ovarian cancer and squamous cell carcinoma [46][47][52,53]. The CXCR4 antagonist GST-NT21MP has been shown to block CXCR4 receptors and inhibit Src activation, thus inhibiting downstream Akt, FAK, and ERK signaling; these events inhibit tumor growth and the metastasis of breast cancer [48][54].
