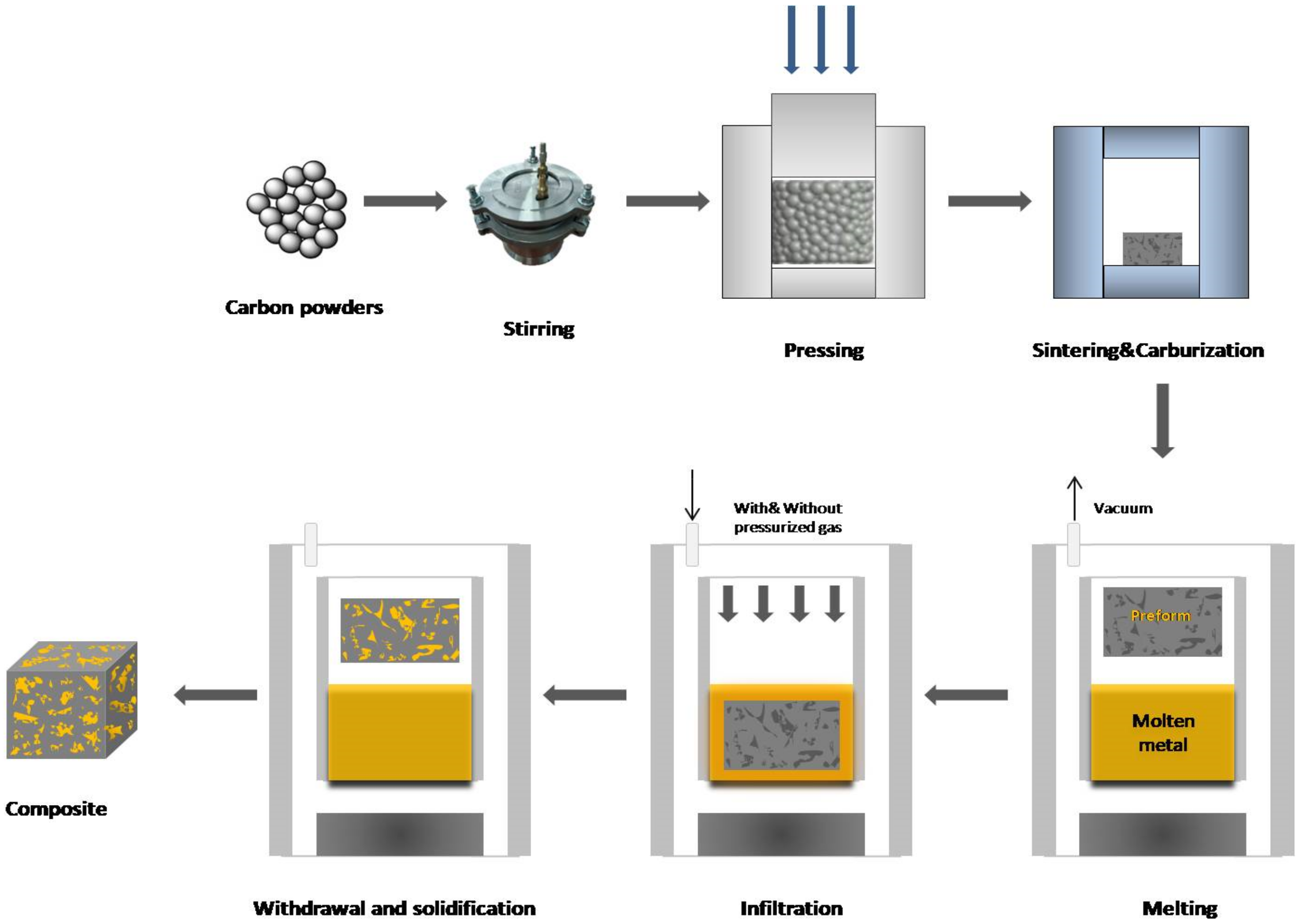
1. Infiltration Technology Preparation of Copper/Carbon Matrix Composites
The method of preparation is based on the infiltration of a prepared carbon preform in an enclosed and heated chamber with molten metal (Figure 1). A preform is usually immersed in a molten matrix metal under a vacuum after the infiltration temperature is reached. Then, the molten metal tends to fill the gaps between the dispersed reinforcing phases. An external pressure (usually inert gas) given to the liquid matrix phase can drive the infiltration process (pressure infiltration), or it can be driven by the capillary forces between the alloy and the dispersed carbon phase (pressureless infiltration).
Figure 1. Gas infiltration process.
In general, the biggest problem encountered during the research is the low wetting angle at the copper–carbon interface and the resulting weak interface bonding. Two methods usually come to the fore to solve this problem. The first is to strengthen the interfacial bond by doping carbide-forming elements into the copper metal alloy
[1]. The second is to strengthen the interfacial bond by making modifications to the carbon preform. For example, Bo Kong
[2] coated carbon preforms with chromium and was able to improve the wettability between carbon and copper surfaces by reducing the contact angle from 140° down to 60°.
In the pressure gas sintering case, in order to ensure that the pore structure of the preform is completely filled and to not hinder the preform deforming, pressures, supported by gas, typically do not exceed 15 MPa
[3] because it is adequate to ensure full pore filling in the structure and that the preform is not subjected to deformation.
The infiltration temperature is usually used as high as possible for the used equipment. A key role is also played by the time of infiltration, thus affecting the reaction of the preform with the alloy via the carbide forming elements present in mixture.
Cu/carbon composite materials for electronics can be manufactured cost effectively using this method. As molten metals (and consequently elevated temperatures) increase the kinetics of interaction between metal and carbon via carbide forming elements, it is significant to take into the account the kinetics of infiltration as well as the chemical protection of the preform if the reaction kinetics are very rapid.
2. Current Stage of the Art of the Infiltration of Copper/Carbon Matrix
This part deals with the recent development in the field of the infiltration of carbon preform with copper. Even though this research focuses on a single method of sample preparation, the infiltration method, some differences still stand out. The most important of these are whether or not the external pressure is used during infiltration. For this reason, this part is examined under two separate chapters. They are further divided by the fact regarding which part (matrix or carbon preform) was modified with carbide forming elements.
In this part, the investigated characteristics and possible industrial applications are also mentioned.
2.1. Infiltration Technologies of Carbon Matrix without Pressure
2.1.1. Addition of Elements into Copper
In order to increase the wettability between the interfaces, the most preferred method is to alloy the copper. According to the literature, titanium is the most prominent as an alloying carbide forming element
[4][5][6][7][8][9][10]. Apart from this, it has been observed that Cr and Si elements are also used
[2][11][12].
Using the melting infiltration process under a vacuum, Yiwen Liu et al.
[4] developed C/Cu composites. First, under a vacuum, the thermosetting phenolic resin was infiltrated into a continuous carbon fiber T300 (PAN) weave fabric in a self-made soak pot. After that, it was pyrolyzed at 773 K under Ar in a carbonization furnace. The processes were carried out twice again. It was possible to produce a porous C-C preform with a density of 1.3 g/cm
3. Under a vacuum, in an electromagnetic induction melting furnace, a Cu alloy containing 1wt.%Cr and 8wt.%Ti was produced. Then, the C-C preform and Cu alloy were placed in the vacuum chamber of the electric furnace. Concerning the melting of the Cu alloy, the materials were heated to 1373 K after evacuation to 10
−3 Pa. Ten minutes later, the alloy was fully melted. It was overheated to 1673 K and kept there for 30 min so that the molten alloy and preform could penetrate entirely with the Cu alloy.
Wetting between the Cu and C-C preform, as well as Cu’s capacity to infiltrate into the C-C preform, was considerably increased by the addition of Ti and Cr to the matrix alloy. Interfacial bonding and high density are hallmarks of the C/Cu composites that have been developed. Ti and C react with carbon fiber in the presence of copper to form TiC. When these composites are exposed to plasma or an electric arc, the cathode spots travel over the surface, resulting in mass loss and degradation of the materials’ properties. To better understand cathode spot behaviour on a carbon fiber reinforced copper matrix composite (C
f/Cu), the same research group
[12] used high-speed digital video cameras and scanning electron microscopes. Vacuum arc erosion is more easily resisted by carbon fibers than by a copper matrix. Instead of following the carbon fibers, the cathode spot travels across the matrix. This type of C
f/Cu has fewer cathode spots than other types of C
f/Cu.
Zhang Huayu and Yiwen Liu
[5] worked with the same materials and process to produce Cu-C composites. They examined the arc erosion resistance of these composites. Under a vacuum atmosphere, the arc erosion rate of a C
f/Cu composite was determined. Infiltration of Cu into the C/C preform was shown to be considerably enhanced by the addition of Ti and Cr, as observed by the outcomes. Fibers and matrix produced a TiC at the interface. Because of this, even after the material had been exposed to plasma, the fiber and matrix remained well-bonded. So, the carbon fibers prevented the composite from being highly eroded as a result of this. Cu, on the other hand, was fully depleted during the arc erosion process. As a result, the graphite had deteriorated to the point where it resembled cauliflower morphology. Because of this, the prepared C
f/Cu was able to withstand significantly higher arc erosion than the usual Cu-C material.
In another study published in 2015, Carlos R Rambo
[6] tried to produce carbon preform via 3D printing. The electrical characteristics and microstructure of dense TiC/Ti-Cu/C composites were investigated. Preforms with 65–78% porosities were infiltrated by 50wt.%Cu-50wt.%Ti at 1373 K with flowing Ar gas.
The process resulted in the development of a composite that was mostly composed of TiC, binary intermetallic Ti-Cu phases, and residual carbon. Given the low electrical resistivity of the samples (ranging from 15 to 60 µΩcm), the newly developed TiC/Ti-Cu/C composites appear to be a very interesting choice for electrical-mechanical applications.
Use of this alloy with porous carbon preforms that can be machined via 3D printing in different shapes can attract new processing technologies with diverse applications, such as EDM electrodes.
Ti element is a good candidate as a dopant for Cu, since it can easily form a carbide layer between carbon and matrix. For this reason, K.X. Zhang et al.
[7] examined the interfacial microstructures that are generated during the infiltration of carbon/carbon (C/C) composites with molten Cu-8wt.%Ti. Before infiltration, the Cu–Ti alloy was placed on top of the C/C preform with a volume ratio of approximately 2:1 between the alloy and the preform, before being infiltrated. After that, the pressureless infiltration procedure was carried out at 1373 K under a vacuum of 10 Pa with a holding time of 30 min. The short-cut fiber web zone has the following two types of interfaces: (i) a combination of fine-grained (FG) TiC and nanosized Cu particles, and (ii) a “Cu layer” with a thickness of max. 500 nm at the pyrolytic carbon (P
yC)/coarse-grained (CG) TiC contact. The forming of the P
yC/TiC and P
yC/Cu
2O interfaces, as well as the P
yC/Cu
2O interface, are the primary mechanisms for the two interfaces’ bonding.
For the purpose of manufacturing the sliding contact material, Cu–Ti alloys were infiltrated into the C/C matrix by Lin Yang
[8]. Preforms made of porous carbon/carbon with a porosity of 20% were immersed in a crucible filled with copper and titanium powders mixed together. In the furnace, they were placed under a vacuum of 10
−2 Pa and infiltration took place at 1573 K for 10 min. Mass fraction of copper within the composite was 28wt.%. There were comparisons of the C/C-Cu composite with a carbon/copper contact strip for the electrical, mechanical and tribological properties, respectively. This material has an advantage over the C/Cu strip in terms of mechanical and electrical properties due to its considerably lower electrical resistivity (0.58 m), better bending strength (186 MPa), along with its 4.7 J/cm
2 impact strength. Compared to the C/Cu strip, the C/C-Cu composite was shown to have superior wear resistance and a lower risk of damaging the copper counterpart. As a result, the C/C-Cu-Ti combination has great promise for use as a new sliding electrical contact material.
Another group, led by Lihui Cui
[9], from Beihang University, also worked on C-C/Cu composites for sliding contacts. In this research, the researchers used carbon fiber 2.5D-braided preforms. Powder Cu-Ti (10wt.%) and C/C preforms with 25% porosity were inserted in the furnace and heated to 1673 K under vacuum pressure for 1 h. As a result, outstanding electrical and mechanical properties were achieved by the C/C-Cu composite’s component phases (TiC), forming an excellent interconnected structure. A compression strength of 324 MPa, flexural strength of 215 MPa, and electrical resistance of 0.63 µΩm proved the composite’s mechanical and electrical superiority over the C/Cu composite strip. Hence, this material might be a good candidate for railway current collector applications.
A Cu–Ti alloy was used in this research
[10]. The ablation properties of the C/C-Cu composite were investigated. Porous C/C composites were encased in a crucible with a mixture of Cu-10wt.%Ti powders. For 30 min, substances were held at 1573 K in a vacuum furnace so as to complete infiltration. The ablation of the Cu and TiC in the composite occurred prior to the mechanical denudation of the C/C composite when the process was carried out under an O
2-C
2H
2 flame. The composite demonstrated superior ablation performance in terms of short-term anti-ablation when compared to a commonly used carbon/carbon composite.
Besides titanium, Si has attracted attention alongside copper and have been used to make the composite brake discs
[11]. A preform was created by hot forming at 170 °C for 30 min at a high temperature. Then, they were pyrolyzed at 2000 °C in an inert gas atmosphere to complete the process. The liquid Si and Cu were infiltrated into the C/C composite after it had been heated to 1823 K under a vacuum. They discovered that the inclusion of copper increased thermal conductivity. Friction coefficients were the more stable in the C/C-SiC-Cu
xSi
y discs than they were in the C/C-SiC discs.
2.1.2. Modification of Carbon Matrix
Although the option to modify the carbon matrix is not as popular as the alloying of the copper metal, some applications are available in the literature for this solution.
Wen Yan Zhou
[13] focused his research on modifying a carbon matrix with Mo
2C to improve the wettability between Cu and C/C. As a result, he published two related articles on this topic. He used an ammonium paramolybdate ((NH
4)
6Mo
24·4H
2O) with a 1:1 molar ratio of sodium chloride and potassium chloride, which was combined to form a flux. Mo
2C coatings were created by burying the C/C preform in the prepared mixture and heating it to 1273 K for 60 min in an alumina crucible. Pure copper was introduced into the Mo
2C-coated C/C preform at a temperature of 1573 K in a vacuum. In one study, the resulting Mo
2C layers had a thickness of about 1 µm and covered the C/C preform’s internal pore surface equally. The non-wettable copper–carbon interface was changed into Mo
2C/carbon and wettable Cu/Mo
2C interfaces by the development of the Mo
2C layer.
In the case of the other study
[14], examination of the microstructure and characteristics of the C/C-Cu composites as a result of graphitization was carried out using two distinct types of preforms (with and without graphitized preforms). Because of the increased degree of graphitization, the C/C-Cu composite with graphitization has reduced electrical resistance, a lower friction coefficient, a lower thermal expansion coefficient, and a significantly higher thermal conductivity. Despite this, the material has lesser flexural strength, impact toughness, and the wear rate was higher. Mostly abrasive wear was observed in the graphitized composite, whereas adhesion wear was observed in the non-graphitized composite. Oxidation wear was observed for both composites.
Reactive melt infiltration (RMI) of the Cu–Ti alloy into porous C/C-SiC composites resulted in the formation of novel friction composites (C/C-Cu
5Si-TiC) with improved friction properties
[15]. Stacks of short fiber webs were needle-punched repeatedly. After that, chemical vapor infiltration (CVI) was used to create porous C/C composites at 1273 K for 120 h in an argon environment with an absolute pressure of 0.1 MPa. H
2 was used as a carrier and diluting gas, while C
3H
6 used as commercial gas. Finally, the C/C-Cu
5Si-TiC composites were prepared by performing a combined LSI (liquid silicon infiltration) and RMI procedure on the porous C/C composites. In order to obtain porous C/C-SiC composites, the LSI procedure was first carried out at 2023 K under a vacuum for 0.5 h. At 1573 K, the C/C-SiC composites were infiltrated by RMI in a vacuum (absolute pressure 1 Pa) for two hours. The RMI process was carried out using a mixture of Cu and Ti powders with a mass ratio of Cu/Ti = 90/10wt.%. The microstructure of the resulting composite revealed that it constituted a Cu
5Si matrix reinforced by TiC particles and intact C/C structures. To put it another way, compared to C/C composites, the C/C-Cu
5Si-TiC composite had better flexural strength, impact resistance, and thermal diffusivity.
2.2. Gas PressureInfiltration of Carbon Matrix
2.2.1. Addition of Elements into Copper
In the gas pressure infiltration methods, many elements, such as Si, B, Fe, are alloyed with copper with various concentrations in recently published papers.
Gionata Schneider and his team
[16] have also worked on Cu infiltration of carbon preforms. The way of response at the interface between a solid porous ceramic and an infiltrating molten metal effects wetting was investigated. In particular, Cu 46 at.% of silicon at 1323–1473 K is required to transform silicon into graphite preforms with 22% porosity. The graphite combines with the silicon in the copper alloy to generate silicon carbide, which may be wetted more effectively by the alloy than the original graphite. According to the results, unlike what has observed in non-reactive systems, metal saturation cannot be stabilized when a constant applied pressure is used. The data can be used to derive an activation volume and an activation energy for the interfacial process that governs the reaction-driven motion of the triple line in this system, with the assumption that applied pressure influences the local rate of thermally activated triple line motion, as applied stress influences the rate of thermally activated dislocation motion.
In another study in Southwest Jiaotong University, China
[17], boron was used as a dopant for Cu. Copper alloying with varying concentrations of the B element to form binary alloys to strengthen the bonding force between carbon and copper was effectively accomplished. Induction melting furnaces were used to make alloys with varying amounts of Cu-B (0, 1.2, and 2.5wt.%). To create the C/Cu composites, gas pressure infiltration was used. Cu–B alloys were cast into a graphite mould, heated to 1423 K under a vacuum of 10
−2 Pa, and held at that temperature for 30 min to guarantee that the substances had melted completely.
A high-purity nitrogen atmosphere was then used to apply a pressure of 5 MPa for 30 min. In the experiments, it was discovered that the electrical conductivity and mechanical strength were proportional to the boron concentration; however, the contact angle was found to be inversely proportional to the amount of boron. Compared to unmodified composites, the flexural strength and electrical conductivity were both improved by 65% and 54%, respectively, when the doping amount was 2.5wt.%. The contact angle was also reduced to 21° when the doping amount was 2.5wt.%. According to the results, the presence of boron carbide at the interface with a modest thickness might strengthen the Cu/C interfacial bonding by converting it from physical bonding to chemical bonding. Boron is shown to increase the wettability of C/Cu systems in this work, leading to improved mechanical and electrical characteristics in Cu-B/sintered-carbon composites.
Previous work
[17] was further expanded by doping 0.6–3.0wt.% boron into copper
[18]. Cu–B (0, 0.6, 1.2, 2.5, and 3.0wt.%) alloys were placed in a graphite mould and heated to 1423 K in a high-temperature infiltration furnace under a vacuum level of 10
−2 Pa for 1 h. Following that, a pressure of 8 MPa was applied in a nitrogen environment for 30 min, and the pressure was maintained throughout the whole process. Based on the results, it can be concluded that the flexural and compressive strengths of the modified composites were raised by 54% and 39%, respectively, and that the C/Cu contact angle fell from 123.6° to 21.3° when 2.5wt.% B doping was used.
Fe element was also one of the metals that was utilized to overcome copper and carbon’s limited wettability
[19]. C/Cu-Fe composites with outstanding characteristics were produced using the gas pressure infiltration approach. The carbon preform and Cu-Fe (0, 3, and 6wt.%) alloy were preheated to 1473 K in a sintering furnace and left to dwell for 2 h before being used. Following complete melting of the Cu alloys, materials were pushed into the carbon substrate in an impregnating tank under a nitrogen pressure of 10 MPa to form a solid bond. The elemental Fe, which was dispersed between the carbon and the copper, reacted with the carbon to generate a Fe
3C layer, which significantly increased the wettability of the carbon/copper combination. The contact angle diminished from 124° to 21° over the course of the experiment. The C/Cu-Fe composites have much higher compressive and flexural strengths when compared to composites without Fe doping, as well as electrical resistivity that is six times greater than the industry standard. Researchers found that introducing Fe
3C into C/Cu composites is a good way to improve the mechanical properties of these materials.
A study held in 2010
[20] focused on C
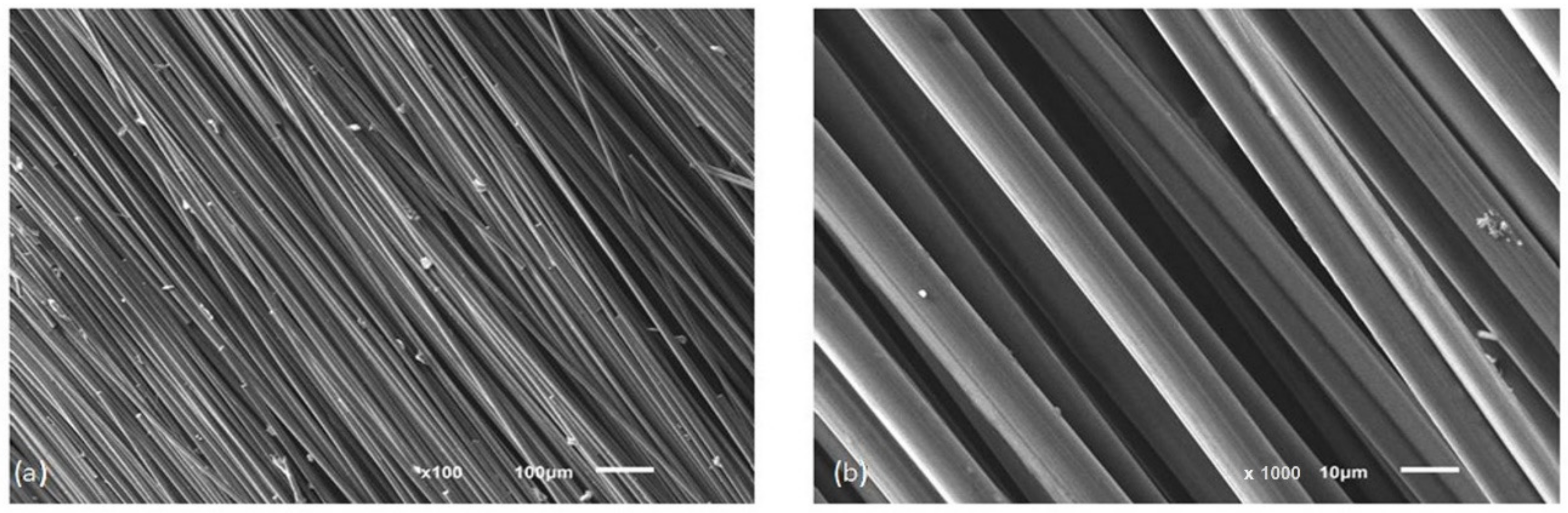
f/Cu composite’s thermal conductivity and thermal expansion measurements in both the longitudinal and transversal directions. Preforms (
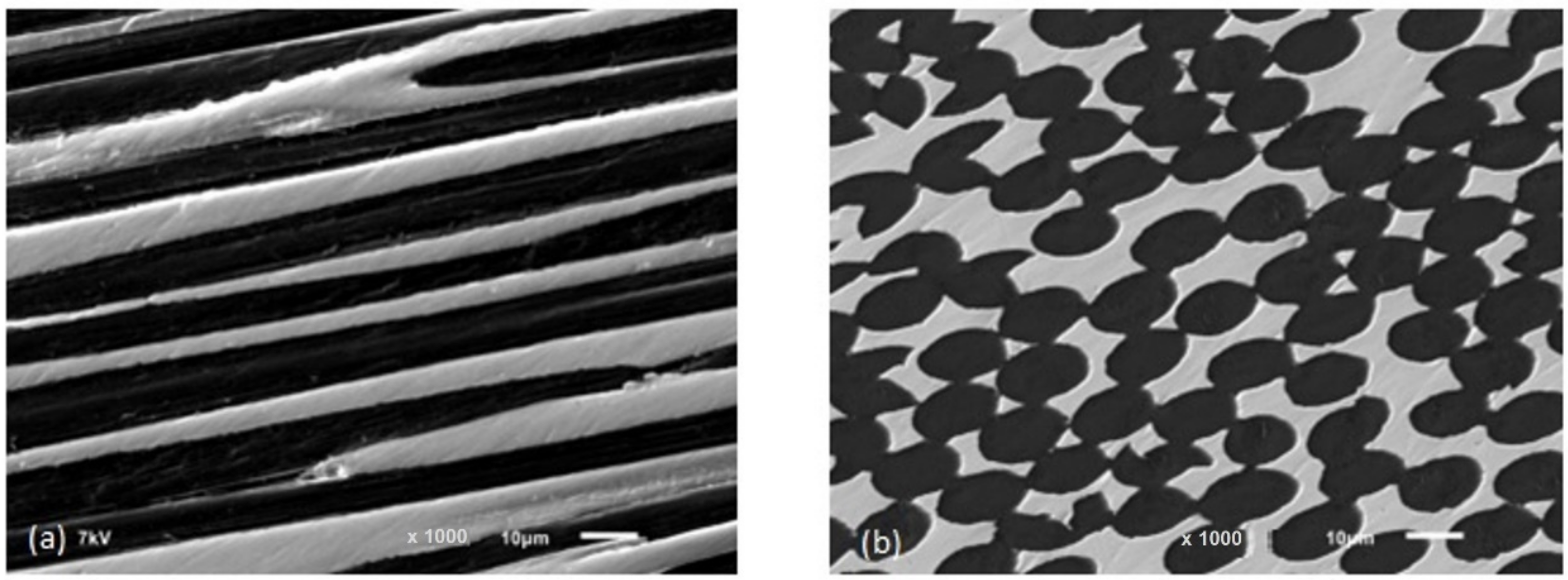
Figure 2) had been placed in a high-pressure autoclave and preheated in a vacuum of 100 Pa before being used in the process. It was necessary to allow approximately 30 min for the system to thermally equilibrate, once the infiltration temperature of 1473 K had been reached. Afterwards, the fibre preforms were immersed in a graphite crucible filled with molten copper to complete the reaction. Nitrogen gas pressure was applied up to 6.0 MPa in 5 min, and the pressure was maintained (
Figure 3). Thermal conductivities were reported as 650 Wm
−1K
−1 in the longitudinal direction and 60.7 Wm
−1K
−1 in the transversal directions, and the mean coefficients of thermal expansion were 0.8 × 10
−6 K
−1 in the longitudinal direction while 23.5 × 10
−6 K
−1 for transversal directions.
Figure 2. SEM images of long carbon fibers K1100 typically used for the preparation of carbon preform: (a) 100× magnification image; (b) 1000× magnification image (IMMS SAS).
Figure 3. SEM microstructure of prepared Cf/Cu composites: (a) longitudinal to fibers; (b) perpendicular to fibers (IMMM SAS).
In a study from Japan, researchers utilized carbon-carbon preforms (C/C) with 20% porosity so as to infiltrate by pure copper
[21]. Liquid copper was added to the low-cost C/C laminate to decrease porosity, enhance oxidation resistance, frictional characteristics, and wear resistance. Perpendicular stacked layers of carbon fibers were sintered via hot press. Then, Cu infiltration into C/C was carried out for around 20 min at temperatures of approximately 1473 K, pressures of 10 MPa, and with Ar gas. Infiltration of liquid copper into the C/C composites had a considerable impact on the tensile strength, stiffness, and fracture toughness of the composites, according to the results of this research.
2.2.2. Modification of Carbon Matrix
Fe2O3 and nickel were introduced into the carbon matrix and composites were obtained with the gas pressure infiltration method.
Ultrafine iron oxide (Fe
2O
3) was added by Wenfu Wei et al.
[22] into the carbon matrix as a precursor in order to increase the interfacial bonding of carbon/copper composites made by pressure infiltration. The interface performance was, therefore, adjusted. The electro-mechanical properties of the impregnated carbon strip were also improved. The carbon matrix with varied Fe
2O
3 content (1wt.%, 3wt.%, 5wt.%, 7wt.%, and 9wt.%) is impregnated with the copper molten solution to produce the C/Cu composite pantograph strip. Compression moulding under 150 MPa with a 5-min dwell time in a cylindrical mould produced carbon powder and active element powder mixed together. Pressed samples were then heated in a carbonization furnace at 1357 K for 120 h, and various types of porous preforms were produced. Infiltration of the carbon matrix was carried out with 18 MPa pressure in the atmosphere, which was created by air and the molten metal was held at 1357 K for 3 min. In addition, a short study on an infiltration simulation in which the carbon preform is doped with WC was included. The composites’ electrical conductivity went up 140.8%, while the compressive and flexural strengths improved by 37.4% and 120.4%, respectively. This work will pave the way for the manufacturing of a new generation of pantograph strips for high-speed train railways.
The same research group led by Wenfu Wei expanded their previous work mentioned above by alloying the copper
[23]. In the experiment, a crucible containing Cu-5wt.% Sn alloy was heated to 1473 K before the Fe
2O
3 doped carbon matrix was added. For 30 min, nitrogen gas from a cylinder was used to enter the carbon matrix. The pressure was set to 12 MPa. Iron carbide (Fe
3C) significantly improved the interfacial bonding between copper and carbon. The contact angle between Cu and carbon was reduced from 124° to 38°, and the wettability was improved. Composites doped with 5wt.% Fe
2O
3 outperformed their unmodified counterparts. The compression and flexural strengths both increased by 37.4% and 120.4%, respectively. Compared to the previous work of the authors
[8], while the mechanical properties remained the same, the electrical conductivity further improved in this research, with an increase of 283.7%.
The composite’s mechanical and electrical characteristics are limited due to the weak interfacial bonding force caused by the inherent non-wetting of the copper and carbon graphite matrix. Qianhua Liao et al.
[24] intend to dope nickel into the carbon matrix to overcome this problem. Different contents of nickel (0wt.%, 1wt.%, 3wt.%, 5wt.%, 7wt.%) were added carbon matrix and then pressed at 150 MPa and sintered at 1300 °C for 2 h to obtain the preform. The Cu-10wt.% Sn alloy was infiltrated at 1523 K for 5 min with 18 MPa pressure into the carbon matrix. The carbon/copper modified composite’s porosity was notably decreased and was joined by a solid solution rather than mechanical interlock, resulting in a much better interfacial condition. The compressive strength and electrical conductivity of the composites were improved by about 52% and 35%, respectively, when the nickel concentration was 3wt.%.
Detailed information about the papers in which the researchers discussed above are given in Table 1 and Table 2.
Table 1. Used starting materials, composition and their properties.
| Preform |
Alloy wt.% |
Preform
Density (g/cm3) |
Pressure and AtmospherePreform
Porosity |
References |
| Infiltration |
Temperature |
Infiltration
Duration |
Density (g/cm3) |
Porosity % |
Interface after Infiltration |
References |
| Porous C-C preform |
Cu-%1 Cr-%8Ti |
1.30 |
- |
| 10−3 Pa under Vacuum |
1400 °C |
30 min | [ | 4 |
7.34] |
| - |
TiC |
[ | 4 | ] |
Porous Cf preform |
Cu-%1 Cr-%8Ti |
1.30 |
- |
[5] |
| Carbon preform |
Cu-%50–Ti-% 50 |
1.80 |
| 10−3 Pa under Vacuum |
1400 °C |
30 min |
- |
- |
TiC |
[5] |
65–78 |
[ |
| Argon atmosphere |
1100 °C | 6 | ] |
| 30 min |
- |
- |
TiC |
[ | 6 | ] |
Porous C-C preform |
Cu-8% Ti |
- |
| 10 Pa under vacuum |
1100 °C | - |
[ | 7] |
| 30 min |
- |
- |
TiC, Cu | 2 | O |
[7] |
Porous C-C preform |
Cu, Ti powder |
- |
20 |
[8] |
| Porous C-C preform |
| 10−2 Pa under vacuum |
1300 °C |
Cu-10% Ti powder |
1.70 |
25 |
[9] |
| 10 min |
2.9 |
- |
TiC |
[ | 8 | ] |
| 10−2 Pa under vacuum |
1400 °C |
1 h |
3.8 |
- |
TiC |
[9] |
Porous C-C preform |
| Vacuum | Cu-10% Ti powder |
1.60 |
6.1 |
1300 °C[10] |
| 30 min |
2.9 |
2.4 |
TiC |
[ | 10 |
C/C preform |
Cu, Si |
1.70 |
- |
[11] |
| ] |
Porous C-C preform |
Cu-%1 Cr-%8Ti |
1.30 |
- |
[12] |
| Mo2C-coated C/Cpreform |
Pure Cu |
1.20 |
- |
[13] |
| Mo2C/C-C preform |
Pure Cu |
1.50 |
- |
[14] |
| Porous C-C/SiC preform |
Cu-10% Ti powder |
1.20 |
37.7 |
[15] |
| Porous Carbon |
Cu-46 at.pct Si |
- |
22 |
[16] |
| Vacuum |
1600 °C |
- |
2.17 |
- |
SiC, Cu3Si |
[ |
Vacuum |
1573 °K |
- |
5.02 |
- |
Mo2C |
[13] |
| Vacuum |
1300 °C |
- |
3.41–4.14 |
- |
Mo2C |
[14] |
| Vacuum pressure < 1 Pa |
1300 °C |
2 h |
3.53–3.66 |
5.8–6.2 |
Cu5Si, TiC, SiC |
[15] |
| 1.1–1.2–1.3–1.4–12 MPa under Ar |
1050 °C–1100 °C–1150 °C–1200 °C |
160–190 min |
- |
- |
SiC, Cu3Si |
[16] |
Carbon preform |
Cu-1.2; 2.5% B |
1.42 |
22.3 |
1.60 |
| 5 MPa under N2 |
1150 °C |
30 min | [ | 17 |
2.79–3.45–3.58] |
| 20.4 |
[ | 19 | ] |
| 2.8–1.3–0.9 |
C fibres |
Pure Cu |
2.20 |
- |
[20] |
| 11 | ] |
B | 4 | C |
[ | 17] |
Carbon preform |
Cu-B (0, 0.6, 1.2, 2.5, and 3.0wt.%) |
1.42 |
22.3 |
[18] |
| 8 MPa under N2 |
1150 °C |
30 min |
2.79–3.58 |
2.8–0.9 |
B4C |
[18] |
| 10 MPa under N2 |
1200 °C |
- |
2.8–3.1–3.3 |
5.2–1.6–1.3 |
Fe3C |
[19] |
| 6 MPa under N2 |
1200 °C |
5 min |
- |
- |
- |
[20] |
C/C preform |
Pure Cu |
1.70 |
20 |
[21] |
| 10 MPa under Ar |
1200 °C |
20 min |
2.68 |
9 |
- |
[21] |
Carbon matrix(%1-3-5-7-9 Fe2O3; % 0.15 WC) |
Pure Cu |
- |
44.35 |
[22] |
| Fe2O3 |
| 10−3 Pa under Vacuum |
1400 °C |
30 min |
- |
- |
TiC |
[12] |
Carbon preform |
Cu-% 0, 3, 6 Fe |
| 18 MPa under Air |
1084 °C |
3 min |
- |
12.1–4.8 |
Fe3C |
[22] |
doped porous C-C preform |
Cu-5% Sn |
1.40–1.48 |
25.2–24.5 |
[23 |
| 12 MPa under N2 |
1200 °C |
30 min |
- | ] |
| 3.51–2.06 |
Fe | 3 | C |
[ | 23 | ] |
Nickel-doped carbon matrix |
Cu-10% Sn |
1.32–1.51 |
33–25 |
[24] |
| 18 MPa/atmosphere unknown |
1250 °C |
5 min |
3.36–3.39 |
- |
Cu9NiSn3 |
[24] |
Cf-Cf preform |
CuSO4 solution |
1.81 |
- |
| Vacuum |
- |
20 min | [ |
1.83–1.86–1.8825] |
Table 2. Infiltration properties of materials in the literature.