Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Aritra Ghosh.
Perovskite solar cells (PSC) have been identified as a game-changer in the world of photovoltaics. This is owing to their rapid development in performance efficiency, increasing from 3.5% to 25.8% in a decade. Further advantages of PSCs include low fabrication costs and high tunability compared to conventional silicon-based solar cells.
- perovskite solar cells
- film fabrication
- commercialization
1. Introduction
The ability to generate electricity from renewable energy sources is of great importance in the fight against climate change. The solar radiation incident on the Earth’s surface is often regarded as the most abundant and safe energy source [1], because the sun provides the Earth’s surface with four million exajoules of solar radiation annually [2]. In fact, one hour of sunlight on Earth provides more energy than what is used in one year, highlighting its importance [3]. The demand for clean energy has grown exponentially over the last decade, particularly in the area of solar photovoltaics (PV). This dramatic growth is shown by an increasing quantity of solar PV installed each year: for the very first time over 100 GW of solar PV was installed globally in the year 2018 [4], and is predicted to reach over 200 GW of newly installed capacity in the year 2022 [5]. Overall, solar PV capacity has now reached a total of 709 GW, representing 24.3% of overall global capacity [6]. The success of solar PV can be attributed to its practicality, low maintenance, and long lifetime [7]. Solar PV can also be installed and implemented in urban environments, unlike other renewable energy sources. This can be in the form of building applied photovoltaic (BAPV) and building integrated photovoltaic (BIPV) [8,9][8][9]. This has led to a huge research effort to improve the efficiency of solar cells to extract as much energy as possible from solar irradiance.
The United Nations sustainable development (UN SDG) goal provides a blueprint to attain a better and sustainable future by 2030. This focuses upon several crucial aspects vital for humanity, among which increment in global percent of energy generation using renewables is one of the agendas. It is aimed to double the global rate of improvement in energy efficiency by 2030. The PV technology is the most efficient renewable energy capacity to meet future energy demands and has dragged the attention of researchers worldwide. Currently, silicon PV technology dominates the market. However, silicon PV technology alone cannot meet the energy demands in the future, and this has urged researchers worldwide to find an alternative and efficient PV technology, dragging the interest of researchers toward PSCs [10].
This inexhaustible resource can be converted into electricity via the photovoltaic effect using a semiconductor material [11]. The general working principle involves electromagnetic radiation from the sun, promoting an electron from the valence band to the conduction band. The energy difference between the valance band and conduction band is known as the bandgap and is a characteristic property of the semiconductor material. This creates electron-hole pairs and if these excited electrons are fed through an external circuit back to the valence band, an electrical current is created [12].
However, there is an upper limit to the power conversion efficiency (PCE) of a single junction solar cell, known as the Shockley–Queisser limit [13]. S. Rühle used this theory to calculate an up-to-date PCE for the global standard spectrum AM 1.5G [14]. The resulting PCE was 33.16% corresponding to a bandgap of 1.34 eV. There has been a lot of research into maximizing the PCE of the active materials used in photovoltaics.
The PV technology is progressing through generations of the cell, namely the first, second and third generations of a cell. The first generation of cells is basically wafer-based cells with thickness ranging from a few 100’s of µm. First-generation solar cells are produced on silicon wafers. These solar cells dominate the market, having a global share of 90% [15]. There are two main types of silicon-based cells: mono-crystalline and polycrystalline. Monocrystalline solar cells are manufactured from single-crystal silicon that is obtained through the Czochralski process [16], which is energy-intensive and expensive. Monocrystalline solar cells are a mature technology and have achieved a PCE of 26.6% [17]. Polycrystalline solar cells are composed of a number of different silicon crystals. The manufacturing and processing costs are lower than for monocrystalline cells, but polycrystalline cells are less efficient.
The PSCs have the capability to attain high efficiency at a low cost as compared to other established cells. PSCs seem to be the potential candidate for attaining high efficiency at low material and low processing costs. The highest advantage that perovskite material holds over conventional PV is its ability to react towards a different range of wavelengths of light, such that maximum incident radiation is converted to electricity. The fact that it can be fabricated over a flexible substrate enables its application in different ways. The PSCs offer the advantage of being lightweight, comprising tailored form factor, easy producible and scalability and many more. Despite being a potential candidate, it is still at the beginning stage of commercialization as compared to other solar technology. The efficient PSCs still contain Au as an electrode which increases the cost of the device. Exploring the low-cost electrode can overcome this shortcoming. Most of the leading PSCs are Pb based, making them toxic in nature. Studies are being conducted to replace the toxic Pb, but still, none of the Pb-free PSCs has defeated the highest performance attained by Pb-based PSCs. The scientific community is actively searching for Pb alternatives to address the issue of toxicity. Metals such as Tin (Sn), germanium (Ge), rubidium (Rb), bismuth (Bi), and antimony (Sb) have all been reported to generate non-toxic or less-toxic metal halide perovskite materials, and their use in PSCs has been proven to be effective [35][18]. Among all the alternatives, Sn has exhibited the best performance. The Sn-based PSCs were lagging in performance as compared to Pb-based PSCs, due to poor stability of the formed perovskite and improper energy level mismatch between the charge transport layer and perovskite. These issues were combated by Nishimura et al. [36][19]. They controlled the A-site cation of the perovskite to regulate the tolerance factor value by one. They partially substituted formamidinium cation (FA) with ethylammonium cation (EA) which not only helped attain the highest PCE of 13% to date but also enhanced the stability of the device. Currently, the most efficient PSCs have attained the best performance by the modification in the charge transport layer and at the interfaces. Min et al. [37][20] placed an interface layer (IL) between ETL and absorber layer. A defect-free connection layer is added. The presence of the IL eliminated the need for passivation. The IL has inherent properties which improve charge carrier transport and extraction from the perovskite layer. This modification also reduced interfacial defects. This work provides us with the guidelines to design minimum defect interfaces between ETL and the perovskite layer. With this modification, a high PCE of 25.5% is obtained with 90% of PCE retained after 500 h of operation. Researchers from the Swiss Federal Institute of Technology Lausanne (EPFL) have enhanced the scalability by replacing ETL with quantum dots. Further high PCE of 25.7% is obtained with high operational stability. The ETL material is replaced by the quantum dots of Tin (IV) oxide. The ETL fabrication had a negative impact on the scalability of the device. Until now, the most widely used ETL material is mesoporous-TiO2. This widely used ETL has the drawback of low electron mobility and also is susceptible to negative photocatalytic events under ultraviolet illumination. The usage of QDs as ETL has enhanced the light trapping efficiency and reduced the charge carrier recombination [38][21]. Azam et al. [39][22] demonstrated the role of interfaces across the perovskite absorber layer and perovskite layer defect passivation on the device performance and stability. They used organic chlorinated salt (benzyl triethylammonium chloride) across the interfaces. This led to better film morphology with proper band alignment across charge transport layers, which rendered considerable improvement in device performance and stability.
2. Issues
The biggest issue facing the commercialization of perovskite solar cells is a lack of stability. These problems arise from the chemical interactions within the perovskite structure. The interactions mostly consist of weak ionic bonds, e.g., the Pb-I bond has a bonding energy of 142 kJmol−1 [115][23]. Other secondary interactions are hydrogen bonding and van der Waals forces, giving the material its soft nature [116][24]. Perovskite solar cells are inherently sensitive to heat, light, moisture, electric fields and oxygen that can all degrade the cell [117][25]. Particular sensitivity to water and moisture was highlighted by Niu et al., concluding that iodide perovskites have a negative standard Gibbs free energy with regards to moisture degradation [118][26]. These stability issues must be addressed in order for perovskite cells to have a long operational life and be commercialized. Further research must be conducted to determine the exact degradation mechanisms, allowing appropriate stabilisation and encapsulation approaches to be developed. Figure 71 summarizes various strategies that can be adopted to improve the device stability along with overall device performance.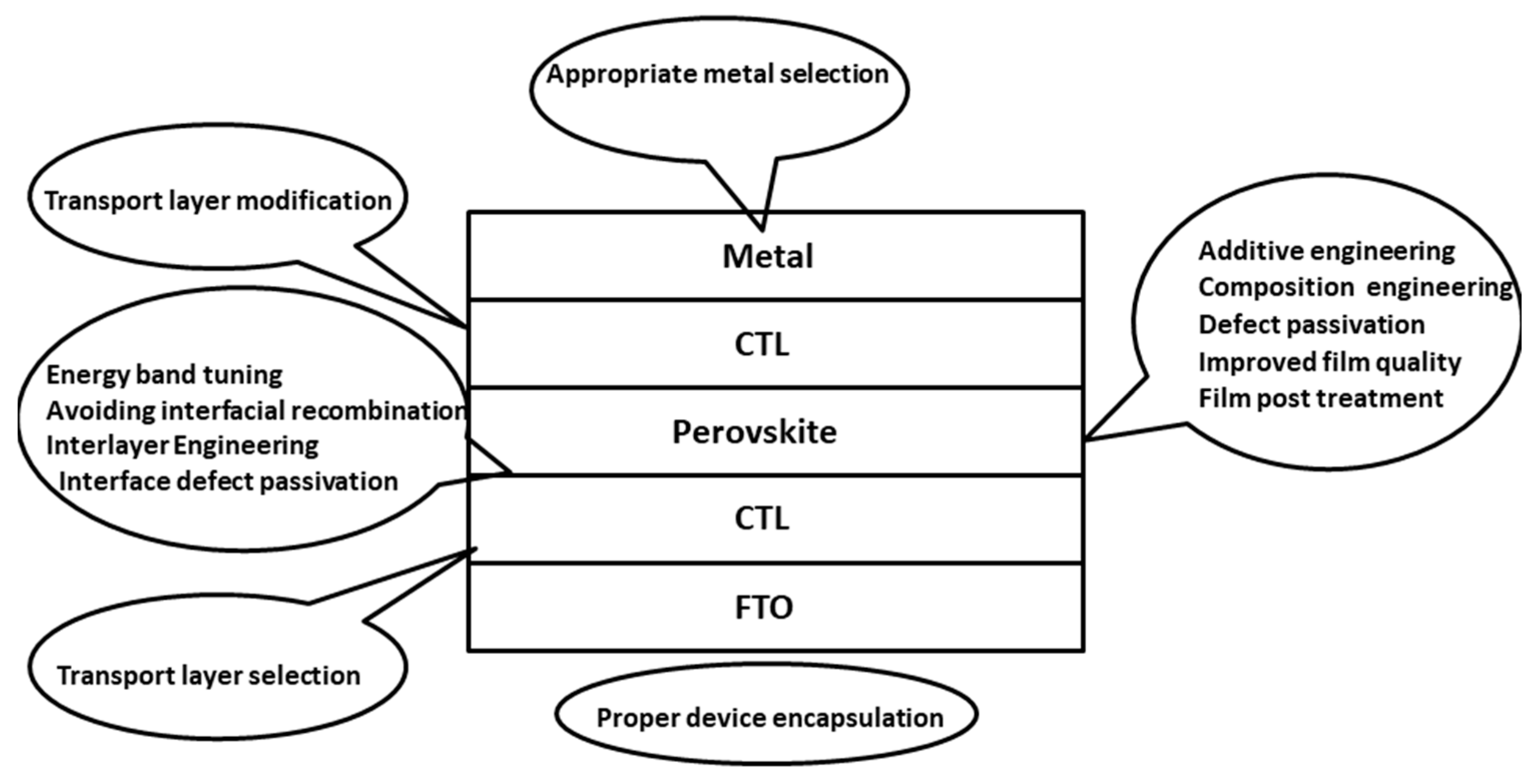
Figure 71. Various strategies to improve stability and avoid degradation in PSCs.
3. Commercialization of PSCs
Long-term device stability is a vital parameter to determine the commercialization of PSCs. The device stability is evaluated in terms of device lifetime tested under 1-sun illumination at electric load. However, PSCs have not attained stability comparable to Si PVs because of their van der Waals interaction causing ion immigration, photo-degradation and phase segregation. Further, the poor intrinsic stability and soft ion lattice due to weak H-bond lead to poor stability. The various commercialization companies are Oxford PV GmbH (Brandenburg, Germany), Swift solar (Sancarlos, CA, USA), Solaronix (Aubonne, Switzerland), Saule Technology (Wroclaw, Poland), Microquanta Semiconductor (Hangzhou, China) etc. [131][39]. Oxford PV developed the world’s first full-size 100MW production line [132][40]. Many commercial companies in China and other countries are working on industrialization of PSCs such as GCL perovskites, Microquanta and a few more. Table 31 summarizes the details of the large-scale PSC modules. One potential issue for perovskite solar cells is the scalability needed for commercialization. The spin-coating method used for the majority of laboratory-scale tests is not effective for producing large-scale uniform coatings. This is because of the lack of consistency in film thickness over a large area, large material waste and lack of compatibility with the roll-to-roll processing that has a high throughput [133,134][41][42]. Industrial-scale techniques such as screen printing and slot-die coating have been identified as the most promising solutions to this [135][43]. They have already been used successfully to fabricate modules over 100 cm2.Table 31.
Details of large-scale PSC modules fabricated by various companies or institutions.
| Company/Institution Name | Country | PCE | Details | Module Area | Reference |
|---|---|---|---|---|---|
| Oxford Photovoltaics |
United Kingdom |
29.52% | Silicon-perovskite bifacial tandem cell | 1 cm2 per cell | [136][44] |
| Kaunas University of Technology | Lithuania | 23.9% | Using spin coating | 26 cm2 active area of module | [137][45] |
| Saule Technology | Poland | 10.2% | Doctor blade coating | 15.7 cm2 flexible module | [138][46] |
| Imec | Belgium | 18.6% | Perovskite solar cell | 16 cm2 module | [139][47] |
| NEDO and Panasonic |
Japan | 16.09% | Using inkjet printing | 802 cm2 module | [140][48] |
| Toshiba and NEDO | Japan | 11.7% | Perovskite solar cell (adjusting crystal growth) | 703 cm2 module | [141][49] |
| Solliance | Netherland | 14.5% | Using the slot die coating | 144 cm2 per cell | [142][50] |
| Chinese Academy of Science (CAS) | China | 19.2% | Using the slot die coating | 16 cm2 module | [143][51] |
| Microquanta | China | 24.1% | Perovskite solar cell | 20 cm2 module | [144][52] |
4. Future Outlook
For more than half a century, silicon PV technology dominates the largest PV market. Studies suggest that in order to obtain the highest efficiency from tandem cells, a wide bandgap perovskite material of bandgap of order 1.7 eV with a thickness of the order of 1 µm must be used. The synthesis of such perovskite material is a hot topic of research among the researchers of the PV community. If a perovskite material of bandgap 1.7 eV is obtained, then extracted voltage will reach about 1.3 V rendering the overall voltage of the device to be 2.0 V. In order to extract similar currents from both the cells, the thickness of the perovskite material must be of order 1 µm with a bandgap of about 1.7 eV. However, preparing high-quality microns-thick perovskite material is still a challenge. Another way to enhance the efficiency of the solar cells is to modify the tunnel junction material [145][53]. The widely used transparent conductive oxide (TCO) is indium tin oxide (ITO). ITOs are not considered ideal due to their improper transmissivity. The ITOs exhibit parasitic absorption at a range of 800 nm [146][54]. An ideal tunnelling junction must have high conductance and high transmittance in order to minimize recombination loss. An appropriate refractive index and thickness are also important to minimize the anisotropic conductance, and internal reflection and avoid lateral breakdown. It can be said that industrialization of tandem cells is completely dependent upon the development of perovskite materials. Currently, state-of-the-art perovskite solar cells still require the use of lead (Pb2+) as the B-cation site. Lead is a toxic element and its use could present problems if released into the environment, eventually working its way into the human food chain [147][55]. Therefore, a large amount of research has been conducted into alternative lead-free perovskite materials. Perovskite solar cells based on different elements such as antimony, copper, germanium, bismuth and others have all been tested [148,149,150,151,152][56][57][58][59][60]. The strongest candidate appears to be tin, having both a similar ionic radius and electronic configuration. This allows direct replacement of the lead ion in the B-site without a significant phase change. Tin-based perovskite cells have a PCE of around 10–12%, which is significantly lower than lead-containing perovskites [153,154][61][62]. It is also important to make sure that environmental burden-shifting is not taking place, as studies have found the oxidation of Sn2+ to Sn4+ can lead to the formation of toxic by-product hydroiodic acid [155][63]. Overall, Ju et al. state that only once the degradation and toxicity mechanisms of current perovskites are understood will lead-free, stable perovskites be fabricated [156][64]. Single-junction perovskite solar cells are not the only technology that has seen a large jump in PCE over the previous decade. Tandem solar cells involving perovskite have been developed and are not constrained to the single-junction Shockley–Quessier limit. The efficiency limit for a tandem solar cell is 47%, much higher than the 31% for single-junction [157,158][65][66]. This is possible as Tandem solar cells better utilize small wavelength radiation from the spectrum. The tandem cell will have a top layer with a large bandgap material; this will absorb the short wavelength part of the spectrum. The longer wavelength radiation will pass through the top layer and be absorbed by the smaller band-gap bottom layer [159][67]. Perovskites can be combined with a variety of materials to create two-terminal tandem cells. Perovskite-Silicon has shown good performance reaching a PCE of 29.15%, outperforming the highest achieving monocrystalline silicon cell [160][68]. Perovskite–Perovskite cells have reached an impressive PCE of 24.8% [161][69]. Another successful combination of materials is perovskite with second-generation material CIGS, achieving 24.2% [162][70]. Overall, the high theoretical efficiency of tandem perovskite solar cells is predicted to allow the price of PV to continue to fall over the coming decades [163][71]. Two-dimensional (2D) Perovskites have also been explored as light absorbers for solar cells due to their wide structural diversity and superior stability compared to conventional 3D perovskites [164][72]. 2D perovskites are produced by using larger ammonium or diammonium cations. These cations are too large and divide the perovskite structure into 2D layers. The layered structures are placed into several categories, with Ruddlesden–Popper (RP) and Dion–Jacobson (DJ) being by far the most common [165,166,167,168][73][74][75][76]. RP structures are stacked so that they have two offset layers per unit cell having pairs of monovalent interlayer spacer cations. DJ structures can be stacked directly on top of each other and only require one divalent interlayer spacer per formula unit [169][77]. A successful example of a RP 2D-perovskite (PEA)2(MA)2[Pb3I10] was synthesized and showed excellent resistance to moisture and allowed high-quality films to be produced after just 1-step [170,171][78][79]. However, this material only achieved a PCE of 4.73% [172][80]. Further improvement of the efficiency was made by improving the charge transport mechanism, reaching a PCE of 12.52% [173][81]. Although, 2D-perovskites show excellent stability they still lack the efficiency of 3D-perovskite cells. For this reason, they are not suitable for single-junction cells but may be an excellent option for use as the high band-gap absorber in a tandem cell [174][82]. The applications of perovskite material in PV involve usage in electric vehicles and building integrated technology because of its flexibility and tunable bandgap [175,176][83][84]. The increment in stability and performance enables the researchers to work not only on performance enhancement but to seek new applications as well. The proper surface engineering and interface engineering improved the efficiency and stability to a great extent. The performance improvement occurred due to a reduction in defect density within the material leading to a decrement in non-radiative recombination. The performance of PSCs is further improved by the surface passivation using hydrophobic molecules. Liang et al. used organic hydrophobic molecules (Benzylamine) with a side chain to enhance the performance of Formamidinium lead iodide (FAPbI3) films. This modification not only enhanced the voltage from 1 to 1.12 V but the stability improved from three days to four months [177,178][85][86]. It can be said that depositing high-quality perovskite film with minimum defects can enhance the performance of the PSCs. Building integration of perovskite-based PV is probably one of the promising approaches. Building energy currently consumes significant energy which must be reduced by employing an energy-positive building envelope [179,180,181,182,183][87][88][89][90][91]. For decades, PV technology is placed in a building in terms of roof integrated or wall integrated technology. These are mainly known as building attached or applied PV. Building integrated type PV application semitransparent PV is a precondition [184,185][92][93]. However, previously most investigation was devoted based on first-generation silicon [186,187,188][94][95][96] or second-generation thin film-based PV [189,190,191][97][98][99]. However, the booming Perovskite industry gives high hope for the building industry [192,193][100][101]. Perovskite which can have variable [194][102] or static [195][103] transparency is the most suitable for building window or façade applications. The recent trend is to include a smart switchable or adaptive window in a building [196,197,198,199,200,201,202,203][104][105][106][107][108][109][110][111]. However, these most effective windows can only reduce energy consumption [204,205,206,207,208][112][113][114][115][116]. In the future, switchable perovskite can be a dominant player in the building industry which can have the potential to generate benign electricity and also tune the transparency concomitantly.References
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269–285.
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900.
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. PNAS 2006, 103, 15729–15735.
- IRENA. Global Energy Transformation: A Roadmap to 2050 (2019 Edition). 2019. Available online: https://www.irena.org/publications/2019/Apr/Global-energy-transformation-A-roadmap-to-20502019Edition?-msclkid=a481d1f3a5fa11ec80aa03b73d80cbce (accessed on 17 March 2022).
- Balkan Green Energy News, Global Solar Power to Cross 200 GW Annual Installation Threshold in 2022. 2021. Available online: https://balkangreenenergynews.com/global-solar-power-to-cross-200-gw-annual-installation-threshold-in-2022/?msclkid=880a2af4a60611ecbf2601d10dd12670 (accessed on 17 March 2022).
- IRENA. Renewable Energy Technologies. 2020. Available online: https://www.irena.org/Statistics/View-Data-by-Topic/Capacity-and-Generation/Technologies (accessed on 17 March 2022).
- Bagher, A.M.; Vahid, M.M.A.; Mohsen, M. Types of Solar Cells and Application. Am. J. Opt. Photonics 2015, 3, 94–113.
- Reddy, P.; Gupta, M.V.N.S.; Nundy, S.; Karthick, A.; Ghosh, A. Status of BIPV and BAPV System for Less Energy-Hungry Building in India—A Review. Appl. Sci. 2020, 10, 2337.
- Ghosh, A. Potential of building integrated and attached/applied photovoltaic (BIPV/BAPV) for adaptive less energy-hungry building’s skin: A comprehensive review. J. Cleaner. Prod. 2020, 276, 123343.
- Envision2030: 17 Goals to Transform the World for Persons with Disabilities. 2022. Available online: https://www.un.org/development/desa/disabilities/envision2030.html (accessed on 29 May 2022).
- Rappaport, P. The photovoltaic effect and its utilisation. Sol. Energy 1959, 3, 8–18.
- Kaushika, N.D.; Mishra, A.; Rai, A.K. Solar Photovoltaics; Springer Nature: Cham, Switzerland, 2019.
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519.
- Rühle, S. Tabulated values if the Shockley-Queisser limit for single junction solar cells. Sol. Energy 2016, 130, 139–147.
- IEA. Technology Roadmap–Solar Photovoltaic Energy 2010. 2010. Available online: https://www.iea.org/reports/technology-roadmap-solar-photovoltaic-energy-2010 (accessed on 22 February 2022).
- Sharma, S.; Jain, K.K.; Sharma, A. Solar Cells: In Research and Applications—A Review. Mater. Sci. Appl. 2015, 6, 1145–1155.
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032.
- Cao, J.; Yan, F. Recent progress in tin-based perovskite solar cells. Energy Environ. Sci. 2021, 14, 1286–1325.
- Nishimura, K.; Kamarudin, M.A.; Hirotani, D.; Hamada, K.; Shen, Q.; Iikubo, S.; Minemoto, T.; Yoshino, K.; Hayase, S. Hayase, Lead-free tin-halide perovskite solar cells with 13% efficiency. Nano Energy 2020, 74, 104858.
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450.
- Quantum Dot Layer Pushes Perovskite Solar Cell Efficiency Up to 25.7%. 2022. Available online: https://www.pv-magazine.com/2022/01/24/quantum-dot-layer-pushes-perovskite-solar-cell-efficiency-up-to-25-7/ (accessed on 26 July 2022).
- Azam, M.; Khan, A.; Liang, G.-X.; Li, G.-J.; Chen, S.; Zheng, Z.-H.; Farooq, U.; Ishaq, M.; Fan, P.; Wang, Z.; et al. Examining the Interfacial Defect Passivation with Chlorinated Organic Salt for Highly Efficient and Stable Perovskite Solar Cells. Sol. RRL 2020, 4, 2000358.
- Li, N.; Niu, X.; Chen, Q.; Zhou, H. Towards commercialization: The operational stability of perovskite solar cells. Chem. Soc. Rev. 2020, 49, 8235–8286.
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The Main Progress of Perovskite Solar Cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152.
- El-Mellouhi, F.; Marzouk, A.; Bentria, E.T.; Rashkeev, S.N.; Kais, S.; Alharbi, F.H. Hydrogen Bonding and Stability of Hybrid Organic-Inorganic Perovskites. ChemSusChem 2016, 9, 2648–2655.
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminium oxide in all-solid-state hybrid solar cells. J. Mater. Chem. 2013, 2, 705–710.
- Lu, H.; Krishna, A.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A. Compositional and Interface Engineering of Organic-Inorganic Lead Halide Perovskite Solar Cells. IScience 2020, 23, 101359.
- Yin, W.-J.; Shi, T.; Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014, 104, 063903.
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cation-Induced Band-Gap Tuning in Organohalide Perovskites: Interplay of Spin–Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616.
- Poespawati, N.R.; Sulistianto, J.; Abuzairi, T.; Purnamaningsih, R.W. Performance and Stability Comparison of Low-Cost Mixed Halide Perovskite Solar Cells: CH3NH3PbI3. Int. J. Photoenergy 2020, 2020, 1–10.
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases observed by coupled thermogravimetry–mass spectrometry analysis. Energy Environ. Sci. 2016, 9, 3406–3410.
- Peng, Z.; Wei, Q.; Chen, H.; Liu, Y.; Wang, F.; Jiang, X.; Liu, W.; Zhou, W.; Ling, S.; Ning, Z. Cs0.15FA0.85PbI3/CsxFA1-xPbI3 Core/Shell Heterostructure for Highly Stable and Efficient Perovskite Solar Cells. Cell Rep. Phys. Sci. 2020, 1, 100224.
- Mazumdar, S.; Du, B.; Huang, C.; Lin, P.; Zhao, J.; Zeng, X.; Ke, S. electron transporting layer for efficient perovskite solar cell by deliberating over nano-electrical conductivity. Sol. Energy Mater. Sol. Cells 2019, 200, 109995.
- Wang, S.; Li, X.; Tong, T.; Han, J.; Zhang, Y.; Zhu, J.; Huang, Z.; Choy, W.C. Sequential Processing: Spontaneous Improvements in Film Quality and Interfacial Engineering for Efficient Perovskite Solar Cells. Sol. RRL 2018, 2, 1800027.
- Prasanna, R.; Gold-Parker, A.; Leijtens, T.; Conings, B.; Babayigit, A.; Boyen, H.G.; Toney, M.F.; McGehee, M.D. Band Gap Tuning via Lattice Contraction and Octahedral Tilting in Perovskite Materials for Photovoltaics. J. Am. Chem. Soc. 2017, 139, 11117–11124.
- Zhang, M.; Chen, Q.; Xue, R.; Zhan, Y.; Wang, C.; Lai, J.; Yang, J.; Lin, H.; Yao, J.; Li, Y.; et al. Reconfiguration of interfacial energy band structure for high-performance inverted structure perovskite solar cells. Nat. Commun. 2019, 10, 4593.
- Mazumdar, S.; Zhao, Y.; Zhang, X. Stability of Perovskite Solar Cells: Degradation Mechanisms and Remedies. Front. Electron. 2021, 2.
- Pearson, A.J.; Eperon, G.E.; Hopkinson, P.E.; Habisreutinger, S.N.; Wang, J.T.; Snaith, H.J.; Greenham, N.C. Oxygen Degradation in Mesoporous Al2O3 /CH3NH3PbI3-xClx Perovskite Solar Cells: Kinetics and Mechanisms. Adv. Energy Mater. 2016, 6, 1600014.
- Liu, H.; Zhang, Z.; Yang, F.; Yang, J.; Grace, A.N.; Li, J.; Tripathi, S.; Jain, S.M. Dopants for enhanced performance of tin-based perovskite solar cells—a short review. Coatings 2021, 11, 1045.
- PV Magazine, Oxford PV Completes 100 MW Factory Build Out. 2021. Available online: https://www.pv-magazine.com/2021/07/23/oxford-pv-completes-100-mw-factory-build-out/ (accessed on 5 July 2022).
- Kim, Y.Y.; Yang, T.Y.; Suhonen, R.; Välimäki, M.; Maaninen, T.; Kemppainen, A.; Jeon, N.J.; Seo, J. Gravure-Printed Flexible Perovskite Solar Cells: Towards Roll-to-Roll Manufacturing. Adv. Sci. 2019, 6, 1802094.
- Dou, B.; Whitaker, J.B.; Bruening, K.; Moore, D.T.; Wheeler, L.M.; Ryter, J.; Breslin, N.J.; Berry, J.J.; Garner, S.M.; Barnes, F.S.; et al. Roll-to-Roll Printing of Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2558–2565.
- Rong, Y.; Ming, Y.; Ji, W.; Li, D.; Mei, A.; Hu, Y. Toward Industrial-Scale Production of Perovskite Solar Cells: Screen Printing, Slot-Die Coating, and Emerging Techniques. J. Phys. Chem. Lett. 2018, 9, 2707–2713.
- Breaking Efficiency Records with Tandem Solar Cells. 2022. Available online: https://www.chemistryworld.com/news/breaking-efficiency-records-with-tandem-solar-cells/4015529.article (accessed on 29 May 2022).
- Liu, C.; Yang, Y.; Rakstys, K.; Mahata, A.; Franckevicius, M.; Mosconi, E.; Skackauskaite, R.; Ding, B.; Brooks, K.G.; Usiobo, O.J.; et al. Tuning structural isomers of phenylenediammonium to afford efficient and stable perovskite solar cells and modules. Nat. Commun. 2021, 12, 6394.
- Researchers at CHOSE and Saule Technologies Design a Large-Area Flexible Perovskite Solar Module Using a Fully Scalable Deposition Technique. 2021. Available online: https://www.perovskite-info.com/researchers-chose-and-saule-technologies-design-large-area-flexible-perovskite (accessed on 29 May 2022).
- Imec Realizes 18.6% Efficient Perovskite Solar Cell. 2021. Available online: https://www.electronicsforu.com/technology-trends/research-papers/imec-realizes-18-6-efficient-perovskite-solar-cell (accessed on 29 May 2022).
- Japan’s NEDO and Panasonic Achieve 16.09% Efficiency for Large-Area Perovskite Solar Cell Module. 2020. Available online: https://www.perovskite-info.com/japan-s-nedo-and-panasonic-achieve-1609-efficiency-large-area-perovskite-solar (accessed on 29 May 2022).
- Saule Technologies on Its Way to Launching Prototype Production Line in Q4 2019. Available online: https://www.perovskite-info.com/saule-technologies-its-way-launching-prototype-production-line-q4-2019 (accessed on 29 May 2022).
- Great Cell Unveils Its Perovskite-Based Solar Cells Commercialization Roadmap 2018. Available online: https://www.perovskite-info.com/greatcell-unveils-its-perovskite-based-solar-cells-commercialization-roadmap (accessed on 29 May 2022).
- Du, M.; Zhu, X.; Wang, L.; Wang, H.; Feng, J.; Jiang, X.; Cao, Y.; Sun, Y.; Duan, L.; Jiao, Y.; et al. High-Pressure Nitrogen-Extraction and Effective Passivation to Attain Highest Large-Area Perovskite Solar Module Efficiency. Adv. Mater. 2020, 32, 2004979.
- Chinese PV Industry Brief: Microquanta Builds 12 MW Ground-Mounted Project with Perovskite Solar Modules. 2022. Available online: https://www.pv-magazine.com/2022/02/18/chinese-pv-industry-brief-microquanta-builds-12-mw-ground-mounted-project-with-perovskite-solar-modules (accessed on 29 May 2022).
- Qiang, Z.; Wang, C.; Gao, X.; Zhao, X.; Tian, H.; Wang, W.; Zong, J.; Fan, J. Challenges of Scalable Development for Perovskite/Silicon Tandem Solar Cells. ACS Appl. Energy Mater. 2022, 5, 6499–6515.
- Albrecht, S.; Saliba, M.; Baena, J.P.C.; Lang, F.; Kegelmann, L.; Mews, M.; Steier, L.; Abate, A.; Rappich, J.; Korte, L.; et al. Monolithic perovskite/silicon-heterojunction tandem solar cells processed at low temperature. Energy Environ. Sci. 2016, 9, 81–88.
- Florence, T.M.; Lilley, S.G.; Stauber, J.L. Skin Absorption of Lead. Lancet 1988, 332, 157–158.
- Saparov, B.; Hong, F.; Sun, J.P.; Duan, H.S.; Meng, W.; Cameron, S. Thin-Film Preparation and Characterisation of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27, 5622–5632.
- Cortecchia, D.; Dewi, H.A.; Yin, J.; Bruno, A.; Chen, S.; Baikie, T.; Boix, P.P.; Grätze, M.; Mhaisalkar, S.; Soci, C.; et al. Lead-Free MA2CuClxBr4-x Hybrid Perovskites. Inorg. Chem. 2016, 55, 1044–1052.
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829–23832.
- Shao, Z.; Mercier, T.L.; Madec, M.B.; Pauporté, T.H. Exploring AgBixI3x+1 semiconductor thin films for lead-free perovskite solar cells. Mater. Des. 2017, 141, 81–87.
- Heydari Gharahcheshmeh, M.; Gleason, K.K. Recent Progress in Conjugated Conducting and Semiconducting Polymers for Energy Devices. Energies 2022, 15, 3661.
- Wu, T.; Liu, X.; He, X.; Wang, Y.; Meng, X.; Noda, T.; Yang, X.; Han, L. Efficient and stable tin-based perovskite cells by introducing π-conjugated Lewis base. Sci. China: Chem. 2019, 63, 107–115.
- Nakamura, T.; Yakumaru, S.; Truong, M.A.; Kim, K.; Liu, J.; Hu, S.; Otsuka, K.; Hashimoto, R.; Murdey, R.; Sasamori, T.; et al. Sn(IV)-free tin perovskite films realized by in situ Sn(0) nanoparticle treatment of the precursor solution. Nat. Commun. 2020, 11, 3008.
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251.
- Ju, M.G.; Chen, M.; Zhou, Y.; Dai, J.; Ma, L.; Padture, N.P.; Zeng, X.C. Towards Eco-friendly and Stable Perovskite Materials for Photovoltaics. Joule 2018, 2, 1231–1241.
- Brown, A.S.; Green, M.A. Detailed balance limit for the series constrained two terminal tandem solar cell. Phys. E 2002, 14, 96–100.
- Bremner, S.P.; Levy, M.Y.; Honsberg, C.B. Analysis of Tandem Solar Cell Efficiencies Under AM1.5G Spectrum Using a Rapid Flux Calculation Method. Prog. Photovolt. Res. Appl. 2007, 16, 225–233.
- Lal, N.N.; Dkhissi, Y.; Li, W.; Hou, Q.; Cheng, Y.B.; Bach, U. Perovskite Tandem Solar Cells. Adv. Energy. Mater. 2017, 7, 1602761.
- Hutchins, M. Tandem Cells Approaching 30% Efficiency. 2020. Available online: https://www.pv-magazine.com/2020/01/30/tandems-cells-approaching-30efficiency/?msclkid=cd9b65a8ab8811ec96d950d8a6454dce (accessed on 24 March 2022).
- Lin, R.; Xiao, K.; Qin, Z.; Han, Q.; Zhang, C.; Wei, M. Monolithic all-perovskite tandem solar cells with 24.8% efficiency exploiting comproportionation to supress Sn(II) oxidation in precursor ink. Nat. Energy. 2019, 4, 864–873.
- Hutchins, M. HZB Hits 23.26% Efficiency with CIGS-Perovskite Tandem Cell. 2019. Available online: https://www.pv-magazine.com/2019/09/11/hzb-hits-23-26-efficiency-with-cigs-perovskite-tandem-cell/ (accessed on 24 March 2022).
- Heo, J.H.; Im, S.H. CH3NH3PbBr3-CH3NH3PbI3 Perovskite-Perovskite Tandem Solar Cells with Exceeding 2.2V Open Circuit Voltage. Adv. Mater. 2015, 28, 5121–5125.
- Li, X.; Hoffman, J.; Ke, W.; Chen, M.; Tsai, H.; Nie, W.; Mohite, A.D.; Kepenekian, M.; Katan, C.; Even, J.; et al. Two-Dimensional Halide Perovskite Incorporating Straight Chain Symmetric Diammonium Ion, (NH3CmH2mNH3)(CH3NH3)n-1PbnI3n+1(m=4–9; n=1–4). J. Am. Chem. Soc. 2018, 140, 12226–12238.
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden-Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867.
- Battle, P.D.; Green, M.A.; Lago, J.; Millburn, J.E.; Rosseinsky, M.J.; Vente, J.F. Crystal and Magnetic Structure of Ca4Mn3O10, an n=3 Ruddlesden-Popper compound. Chem. Mater. 1998, 10, 658–664.
- Dion, M.; Ganne, M.; Tournoux, M. Nouvelles familles de phases MIMII2Nb3O10 a feuillets “Perovskites”. Mater. Res. Bull. 1981, 16, 1429–1435.
- Hojamberdiev, M.; Bekheet, M.F.; Zahedi, E.; Wagata, H.; Kamei, Y.; Yubuta, K.; Gurlo, A.; Matsushita, N.; Domen, K.; Teshima, K.; et al. New Dion-Jacobson Phase Three-Layer Perovskite CsBa2Ta3O10 and Its Conversion to Nitrided Ba2Ta3O10 Nanosheets via a Nitridation-Protonation-Intercalation-Exfoliation Route for Water Splitting. Cryst. Growth Des. 2016, 16, 2302–2308.
- Mao, L.; Ke, W.; Pedesseau, L.; Wu, Y.; Katan, C.; Even, J.; Wasielewski, M.R.; Stoumpos, C.C.; Kanatzidis, M. Hybrid Dion-Jacobson 2D Lead Iodide Structures. J. Am. Chem. Soc. 2018, 140, 3775–3783.
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D Homologous Perovskites as Light-Absorbing Materials for Solar Cell Applications. J. Am. Chem. Soc. 2015, 137, 7843–7850.
- Kagan, C.R.; Mitzi, D.B.; Dimitrakopoulos, C.D. Organic-Inorganic Hybrid Materials as Semiconducting Channels in Thin-Film Field-Effect Transitions. Science 1999, 286, 945–947.
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235.
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden-Popper Perovskite Solar Cells. Nature 2016, 536, 312–316.
- Vos, A.D. Detailed balance limit of the efficiency of tandem solar cells. J. Phys. D: Appl. Phys. 1980, 13, 839–846.
- Ghosh, A. Possibilities and Challenges for the Inclusion of the Electric Vehicle (EV) to Reduce the Carbon Footprint in the Transport Sector: A Review. Energies 2020, 13, 2602.
- Ghosh, A.; Norton, B. Optimization of PV powered SPD switchable glazing to minimise probability of loss of power supply. Renew. Energy 2019, 131, 993–1001.
- Wu, G.; Liang, R.; Ge, M.; Sun, G.; Zhang, Y.; Xing, G. Surface Passivation Using 2D Perovskites toward Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2022, 34, 2105635.
- Hoex, B. Functional Thin Films for High-Efficiency Solar Cells. PhD Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2008.
- Nundy, S.; Ghosh, A.; Mesloub, A.; Noaime, E.; Touahmia, M. Comfort Analysis of Hafnium (Hf) Doped ZnO Coated Self-Cleaning Glazing for Energy-Efficient. Materials 2022, 15, 4934.
- Nundy, S.; Ghosh, A. Thermal and visual comfort analysis of adaptive vacuum integrated switchable suspended particle device window for temperate climate. Renew. Energy 2020, 156, 1361–1372.
- Nundy, S.; Mesloub, A.; Alsolami, B.M.; Ghosh, A. Electrically actuated visible and near-infrared regulating switchable smart window for energy positive building: A review. J. Clean. Prod. 2021, 301, 126854.
- Nundy, S.; Ghosh, A.; Tahir, A.; Mallick, T.K. Role of Hafnium Doping on Wetting Transition Tuning the Wettability Properties of ZnO and Doped Thin Films: Self-Cleaning Coating for Solar Application. ACS Appl. Mater. Interfaces 2021, 13, 25540–25552.
- Nundy, S.; Ghosh, A.; Mallick, T.K. Hydrophilic and Superhydrophilic Self-Cleaning Coatings by Morphologically Varying ZnO Microstructures for Photovoltaic and Glazing Applications. ACS Omega 2020, 5, 1033–1039.
- Selvaraj, P.; Ghosh, A.; Mallick, T.K.; Sundaram, S. Investigation of semi-transparent dye-sensitized solar cells for fenestration integration. Renew. Energy 2019, 141, 516–525.
- Ghosh, A.; Selvaraj, P.; Sundaram, S.; Mallick, T.K. The colour rendering index and correlated colour temperature of dye-sensitized solar cell for adaptive glazing application. Sol. Energy 2018, 163, 537–544.
- Ghosh, A.; Sundaram, S.; Mallick, T.K. Colour properties and glazing factors evaluation of multicrystalline based semi-transparent Photovoltaic-vacuum glazing for BIPV application. Renew. Energy 2019, 131, 730–736.
- Ghosh, A.; Sarmah, N.; Sundaram, S.; Mallick, T.K. Numerical studies of thermal comfort for semi-transparent building integrated photovoltaic (BIPV) -vacuum glazing system. Sol. Energy 2019, 190, 608–616.
- Ghosh, A.; Sundaram, S.; Mallick, T.K. Investigation of thermal and electrical performances of a combined semi-transparent PV-vacuum glazing. Appl. Energy 2018, 228, 1591–1600.
- Alrashidi, H.; Ghosh, A.; Issa, W.; Sellami, N.; Mallick, T.K.; Sundaram, S. Thermal performance of semitransparent CdTe BIPV window at temperate climate. Sol. Energy 2020, 195, 536–543.
- Alrashidi, H.; Ghosh, A.; Issa, W.; Sellami, N.; Mallick, T.K.; Sundaram, S. Evaluation of solar factor using spectral analysis for CdTe photovoltaic glazing. Mater. Lett. 2019, 237, 332–335.
- Alrashidi, H.; Issa, W.; Sellami, N.; Ghosh, A.; Mallick, T.K.; Sundaram, S. Performance assessment of cadmium telluride-based semi-transparent glazing for power saving in façade buildings. Energy Build. 2020, 215, 109585.
- Ghosh, A.; Mesloub, A.; Touahmia, M.; Ajmi, M. Visual Comfort Analysis of Semi-Transparent Perovskite Based Building Integrated Photovoltaic Window for Hot Desert. Energies 2021, 14, 1043.
- Ghosh, A.; Bhandari, S.; Sundaram, S.; Mallick, T.K. Carbon counter electrode mesoscopic ambient processed & characterised perovskite for adaptive BIPV fenestration. Renew. Energy 2020, 145, 2151–2158.
- Roy, A.; Ullah, H.; Ghosh, A.; Baig, H.; Sundaram, S.; Tahir, A.A.; Mallick, T.K. Understanding the Semi-Switchable Thermochromic Behavior of Mixed Halide Hybrid Perovskite Nanorods. J. Phys. Chem. C 2021, 125, 18058–18070.
- Bhandari, S.; Ghosh, A.; Roy, A.; Kumar, T.; Sundaram, S. Compelling temperature behaviour of carbon-perovskite solar cell for fenestration at various climates. Chem. Eng. J. Adv. 2022, 10, 100267.
- Shaik, S.; Nundy, S.; Ramana, V.; Ghosh, A.; Afzal, A. Polymer dispersed liquid crystal retrofitted smart switchable glazing: Energy saving, diurnal illumination, and CO2 mitigation prospective. J. Clean. Prod. 2022, 350, 131444.
- Mesloub, A.; Ghosh, A.; Touahmia, M.; Abdullah, G.; Alsolami, B.M.; Ahriz, A. Assessment of the overall energy performance of an SPD smart window in a hot desert climate The International Commission on Illumination. Energy 2022, 252, 124073.
- Chidubem Iluyemi, D.; Nundy, S.; Shaik, S.; Tahir, A.; Ghosh, A. Building energy analysis using EC and PDLC based smart switchable window in Oman. Sol. Energy 2022, 237, 301–312.
- Ghosh, A.; Norton, B.; Duffy, A. Measured overall heat transfer coefficient of a suspended particle device switchable glazing. Appl. Energy 2015, 159, 362–369.
- Ghosh, A.; Norton, B.; Duffy, A. Behaviour of a SPD switchable glazing in an outdoor test cell with heat removal under varying weather conditions. Appl. Energy 2016, 180, 695–706.
- Ghosh, A.; Norton, B.; Duffy, A. Measured thermal performance of a combined suspended particle switchable device evacuated glazing. Appl. Energy 2016, 169, 469–480.
- Ghosh, A.; Norton, B.; Duffy, A. Measured thermal & daylight performance of an evacuated glazing using an outdoor test cell. Appl. Energy 2016, 177, 196–203.
- Ghosh, A.; Norton, B.; Duffy, A. First outdoor characterisation of a PV powered suspended particle device switchable glazing. Sol. Energy Mater. Sol. Cells 2016, 157, 1–9.
- Ghosh, A.; Norton, B.; Duffy, A. Effect of sky clearness index on transmission of evacuated (vacuum) glazing. Renew. Energy 2017, 105, 160–166.
- Ghosh, A.; Norton, B.; Duffy, A. Effect of sky conditions on light transmission through a suspended particle device switchable glazing. Sol. Energy Mater. Sol. Cells 2017, 160, 134–140.
- Ghosh, A.; Norton, B.; Duffy, A. Effect of atmospheric transmittance on performance of adaptive SPD-vacuum switchable glazing. Sol. Energy Mater. Sol. Cells 2017, 161, 424–431.
- Ghosh, A.; Norton, B. Durability of switching behaviour after outdoor exposure for a suspended particle device switchable glazing. Sol. Energy Mater. Sol. Cells 2017, 163, 178–184.
- Ghosh, A.; Norton, B. Interior colour rendering of daylight transmitted through a suspended particle device switchable glazing. Sol. Energy Mater. Sol. Cells 2017, 163, 218–223.
More
