Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Aritra Ghosh and Version 3 by Catherine Yang.
Perovskite solar cells (PSC) have been identified as a game-changer in the world of photovoltaics. This is owing to their rapid development in performance efficiency, increasing from 3.5% to 25.8% in a decade. Further advantages of PSCs include low fabrication costs and high tunability compared to conventional silicon-based solar cells.
- perovskite solar cells
- film fabrication
- commercialization
1. Introduction
The ability to generate electricity from renewable energy sources is of great importance in the fight against climate change. The solar radiation incident on the Earth’s surface is often regarded as the most abundant and safe energy source [1], because the sun provides the Earth’s surface with four million exajoules of solar radiation annually [2]. In fact, one hour of sunlight on Earth provides more energy than what is used in one year, highlighting its importance [3]. The demand for clean energy has grown exponentially over the last decade, particularly in the area of solar photovoltaics (PV). This dramatic growth is shown by an increasing quantity of solar PV installed each year: for the very first time over 100 GW of solar PV was installed globally in the year 2018 [4], and is predicted to reach over 200 GW of newly installed capacity in the year 2022 [5]. Overall, solar PV capacity has now reached a total of 709 GW, representing 24.3% of overall global capacity [6]. The success of solar PV can be attributed to its practicality, low maintenance, and long lifetime [7]. Solar PV can also be installed and implemented in urban environments, unlike other renewable energy sources. This can be in the form of building applied photovoltaic (BAPV) and building integrated photovoltaic (BIPV) [8][9][8,9]. This has led to a huge research effort to improve the efficiency of solar cells to extract as much energy as possible from solar irradiance.
The United Nations sustainable development (UN SDG) goal provides a blueprint to attain a better and sustainable future by 2030. This focuses upon several crucial aspects vital for humanity, among which increment in global percent of energy generation using renewables is one of the agendas. It is aimed to double the global rate of improvement in energy efficiency by 2030. The PV technology is the most efficient renewable energy capacity to meet future energy demands and has dragged the attention of researchers worldwide. Currently, silicon PV technology dominates the market. However, silicon PV technology alone cannot meet the energy demands in the future, and this has urged researchers worldwide to find an alternative and efficient PV technology, dragging the interest of researchers toward PSCs [10].
This inexhaustible resource can be converted into electricity via the photovoltaic effect using a semiconductor material [11]. The general working principle involves electromagnetic radiation from the sun, promoting an electron from the valence band to the conduction band. The energy difference between the valance band and conduction band is known as the bandgap and is a characteristic property of the semiconductor material. This creates electron-hole pairs and if these excited electrons are fed through an external circuit back to the valence band, an electrical current is created [12].
However, there is an upper limit to the power conversion efficiency (PCE) of a single junction solar cell, known as the Shockley–Queisser limit [13]. S. Rühle used this theory to calculate an up-to-date PCE for the global standard spectrum AM 1.5G [14]. The resulting PCE was 33.16% corresponding to a bandgap of 1.34 eV. There has been a lot of research into maximizing the PCE of the active materials used in photovoltaics.
The PV technology is progressing through generations of the cell, namely the first, second and third generations of a cell. The first generation of cells is basically wafer-based cells with thickness ranging from a few 100’s of µm. First-generation solar cells are produced on silicon wafers. These solar cells dominate the market, having a global share of 90% [15]. There are two main types of silicon-based cells: mono-crystalline and polycrystalline. Monocrystalline solar cells are manufactured from single-crystal silicon that is obtained through the Czochralski process [16], which is energy-intensive and expensive. Monocrystalline solar cells are a mature technology and have achieved a PCE of 26.6% [17]. Polycrystalline solar cells are composed of a number of different silicon crystals. The manufacturing and processing costs are lower than for monocrystalline cells, but polycrystalline cells are less efficient.
The PSCs have the capability to attain high efficiency at a low cost as compared to other established cells. PSCs seem to be the potential candidate for attaining high efficiency at low material and low processing costs. The highest advantage that perovskite material holds over conventional PV is its ability to react towards a different range of wavelengths of light, such that maximum incident radiation is converted to electricity. The fact that it can be fabricated over a flexible substrate enables its application in different ways. The PSCs offer the advantage of being lightweight, comprising tailored form factor, easy producible and scalability and many more. Despite being a potential candidate, it is still at the beginning stage of commercialization as compared to other solar technology. The efficient PSCs still contain Au as an electrode which increases the cost of the device. Exploring the low-cost electrode can overcome this shortcoming. Most of the leading PSCs are Pb based, making them toxic in nature. Studies are being conducted to replace the toxic Pb, but still, none of the Pb-free PSCs has defeated the highest performance attained by Pb-based PSCs. The scientific community is actively searching for Pb alternatives to address the issue of toxicity. Metals such as Tin (Sn), germanium (Ge), rubidium (Rb), bismuth (Bi), and antimony (Sb) have all been reported to generate non-toxic or less-toxic metal halide perovskite materials, and their use in PSCs has been proven to be effective [18][35]. Among all the alternatives, Sn has exhibited the best performance. The Sn-based PSCs were lagging in performance as compared to Pb-based PSCs, due to poor stability of the formed perovskite and improper energy level mismatch between the charge transport layer and perovskite. These issues were combated by Nishimura et al. [19][36]. They controlled the A-site cation of the perovskite to regulate the tolerance factor value by one. They partially substituted formamidinium cation (FA) with ethylammonium cation (EA) which not only helped attain the highest PCE of 13% to date but also enhanced the stability of the device. Currently, the most efficient PSCs have attained the best performance by the modification in the charge transport layer and at the interfaces. Min et al. [20][37] placed an interface layer (IL) between ETL and absorber layer. A defect-free connection layer is added. The presence of the IL eliminated the need for passivation. The IL has inherent properties which improve charge carrier transport and extraction from the perovskite layer. This modification also reduced interfacial defects. This work provides us with the guidelines to design minimum defect interfaces between ETL and the perovskite layer. With this modification, a high PCE of 25.5% is obtained with 90% of PCE retained after 500 h of operation. Researchers from the Swiss Federal Institute of Technology Lausanne (EPFL) have enhanced the scalability by replacing ETL with quantum dots. Further high PCE of 25.7% is obtained with high operational stability. The ETL material is replaced by the quantum dots of Tin (IV) oxide. The ETL fabrication had a negative impact on the scalability of the device. Until now, the most widely used ETL material is mesoporous-TiO2. This widely used ETL has the drawback of low electron mobility and also is susceptible to negative photocatalytic events under ultraviolet illumination. The usage of QDs as ETL has enhanced the light trapping efficiency and reduced the charge carrier recombination [21][38]. Azam et al. [22][39] demonstrated the role of interfaces across the perovskite absorber layer and perovskite layer defect passivation on the device performance and stability. They used organic chlorinated salt (benzyl triethylammonium chloride) across the interfaces. This led to better film morphology with proper band alignment across charge transport layers, which rendered considerable improvement in device performance and stability.
2. Issues
The biggest issue facing the commercialization of perovskite solar cells is a lack of stability. These problems arise from the chemical interactions within the perovskite structure. The interactions mostly consist of weak ionic bonds, e.g., the Pb-I bond has a bonding energy of 142 kJmol−1 [23][115]. Other secondary interactions are hydrogen bonding and van der Waals forces, giving the material its soft nature [24][116]. Perovskite solar cells are inherently sensitive to heat, light, moisture, electric fields and oxygen that can all degrade the cell [25][117]. Particular sensitivity to water and moisture was highlighted by Niu et al., concluding that iodide perovskites have a negative standard Gibbs free energy with regards to moisture degradation [26][118]. These stability issues must be addressed in order for perovskite cells to have a long operational life and be commercialized. Further research must be conducted to determine the exact degradation mechanisms, allowing appropriate stabilisation and encapsulation approaches to be developed. Figure 17 summarizes various strategies that can be adopted to improve the device stability along with overall device performance.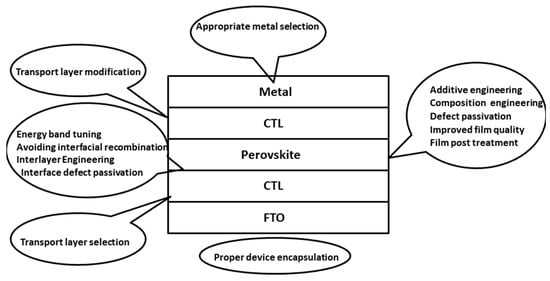
Figure 17. Various strategies to improve stability and avoid degradation in PSCs.
3. Commercialization of PSCs
Long-term device stability is a vital parameter to determine the commercialization of PSCs. The device stability is evaluated in terms of device lifetime tested under 1-sun illumination at electric load. However, PSCs have not attained stability comparable to Si PVs because of their van der Waals interaction causing ion immigration, photo-degradation and phase segregation. Further, the poor intrinsic stability and soft ion lattice due to weak H-bond lead to poor stability. The various commercialization companies are Oxford PV GmbH (Brandenburg, Germany), Swift solar (Sancarlos, CA, USA), Solaronix (Aubonne, Switzerland), Saule Technology (Wroclaw, Poland), Microquanta Semiconductor (Hangzhou, China) etc. [39][131]. Oxford PV developed the world’s first full-size 100MW production line [40][132]. Many commercial companies in China and other countries are working on industrialization of PSCs such as GCL perovskites, Microquanta and a few more. Table 13 summarizes the details of the large-scale PSC modules. One potential issue for perovskite solar cells is the scalability needed for commercialization. The spin-coating method used for the majority of laboratory-scale tests is not effective for producing large-scale uniform coatings. This is because of the lack of consistency in film thickness over a large area, large material waste and lack of compatibility with the roll-to-roll processing that has a high throughput [41][42][133,134]. Industrial-scale techniques such as screen printing and slot-die coating have been identified as the most promising solutions to this [43][135]. They have already been used successfully to fabricate modules over 100 cm2.Table 13.
Details of large-scale PSC modules fabricated by various companies or institutions.
| Company/Institution Name | Country | PCE | Details | Module Area | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oxford | Photovoltaics | United Kingdom |
29.52% | Silicon-perovskite bifacial tandem cell | 1 cm | 2 | per cell | [44] | [136] | |
| Kaunas University of Technology | Lithuania | 23.9% | Using spin coating | 26 cm | 2 | active area of module | [45] | [137] | ||
| Saule Technology | Poland | 10.2% | Doctor blade coating | 15.7 cm | 2 | flexible module | [46] | [138] | ||
| Imec | Belgium | 18.6% | Perovskite solar cell | 16 cm | 2 | module | [47] | [139] | ||
| NEDO and | Panasonic | Japan | 16.09% | Using inkjet printing | 802 cm | 2 | module | [48] | [140] | |
| Toshiba and NEDO | Japan | 11.7% | Perovskite solar cell (adjusting crystal growth) | 703 cm | 2 | module | [49] | [141] | ||
| Solliance | Netherland | 14.5% | Using the slot die coating | 144 cm | 2 | per cell | [50] | [142] | ||
| Chinese Academy of Science (CAS) | China | 19.2% | Using the slot die coating | 16 cm | 2 | module | [51] | [143] | ||
| Microquanta | China | 24.1% | Perovskite solar cell | 20 cm | 2 | module | [52] | [144] |
