Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Julian Louis Muff and Version 2 by Jessie Wu.
Short bowel syndrome (SBS) in children is defined as “the need for parenteral nutrition (PN) for >60 days after intestinal resection or a bowel length of less than 25% of expected” by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN).
- short bowel syndrome
- parenteral nutrition
- antimotility agents
1. Definitions
Short bowel syndrome reflects the most common cause for intestinal failure (IF), which is defined by the European Society for Clinical Nutrition and Metabolism (ESPEN) as “the reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, such that intravenous supplementation is required to maintain health and/or growth” [1][2]. Other causes of IF include intestinal dysmotility, intestinal fistula, mechanical obstruction and extensive small bowel mucosal disease [1][2]. SBS remains a clinical diagnosis. In adults, SBS has typically been diagnosed when a patient has a total functional small bowel length of less than 200 cm with requirement for nutritional and/or fluid substitution [2][3]. In children, a clear definition of short bowel in terms of length in absolute numbers has not been made, likely due to the fact that pediatric intestinal length is linked to the state of growth [1][2]. However, in 1967 Rickham was the first to attempt to define short bowel as a remaining intestine of 30% of normal length, or in absolute numbers less than 75 cm, in a full-term neonate [3][4]. In children, SBS can be further categorized into: “very SBS” (≤40 cm), “ultra SBS” (between <30 cm and <10 cm) and “no gut syndrome” (only duodenum is left) [4][5][5,6].
The length of the small intestine of adults measured from the ligament of Treitz to the ileocecal valve ranges from about 275 cm to 850 cm [2][3], with an average of about 360 cm [6][7]. Another study based on human adult cadavers reports average small intestinal lengths as 670 cm in males and 599 cm in females [7][8]. According to a cadaver study by Touloukian and Smith in term infants, the average small intestinal length is reported to be around 250 cm. During fetal development, the small intestine measures approximately 120 cm in the second trimester and doubles during the third trimester [8][9]. Another more recent study by Strujis et al. in regard to small bowel length measured in vivo states with typical lengths of 100 cm at a gestational age of 27–29 weeks, 160 cm at 39–40 weeks and 240 cm between zero and six months of age, and they showed that small bowel length is best correlated with body length [9][10]. An overview of average small bowel lengths in various (gestational) age groups is provided in Figure 1. In general, there are great inconsistencies in the description of normal small bowel lengths in the literature.
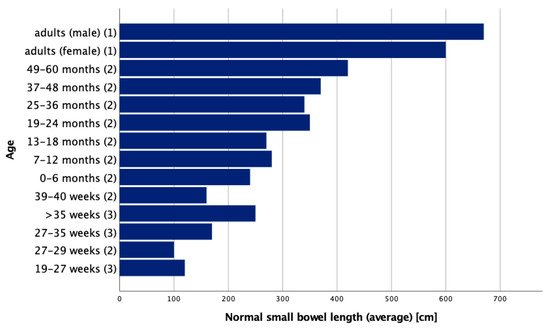
Figure 1.
Normal small bowel lengths in various ages adapted from (
1
) Hounnou et al.; (
2
) Struijs et al.; (
SBS may result from congenital or acquired conditions. Mostly, SBS results from extensive surgical resection of the small intestine due to midgut volvulus, malrotation, extensive aganglionosis or necrotizing enterocolitis [10][11], the latter reflecting the most common cause of bowel resection in children (29% of cases) [11][12]. In adults, multiple small bowel resections related to recurrent Crohn’s disease or massive resections due to catastrophic mesenteric events (arterial embolism, venous thrombosis), trauma or malignancies represent the most common causes of SBS [10][12][11,13]. A comprehensive overview of causes of small intestinal resection in children and adults is provided in Table 1.
Table 1. Overview of causes of small bowel syndrome in children and adults. Adapted from Buchman and DiBaise et al. [10][12].
Overview of causes of small bowel syndrome in children and adults. Adapted from Buchman and DiBaise et al. [11,13].
| Children | Adults | |
|---|---|---|
| Congenital | Jejunal atresia Ileal atresia |
- |
| Acquired (extensive small bowel resection) | Necrotizing enterocolitis Malrotation with midgut volvulus Gastroschisis Extensive aganglionosis Trauma |
Crohn’s disease Catastrophic mesenteric events (arterial embolism, venous thrombosis) Volvulus Trauma Adhesive obstruction Malignancies Radiation enteritis |
| Functional SBS in severe malabsorption (bowel length intact) | Chronic intestinal pseudo-obstruction syndrome Refractory sprue Radiation enteritis Congenital villous atrophy |
|
The incidence of SBS is reported by a Canadian study as 24.5 per 100,000 live births, and is much greater in premature births (353.7 per 100,000) [13][14]. Exact data on the prevalence of SBS do not exist. Data on home parenteral nutrition (HPN) are used as a proxy to estimate the prevalence of SBS. An European multicenter study from 1999 reports a point prevalence of four per million inhabitants, 35% of which received HPN because of SBS [14][15]. Another study reports the annual prevalence of HPN in the United States as one-hundred and twenty per million, approximately a quarter receiving HPN due to SBS [15][16]. DiBaise et al. state that these numbers seem to be an underestimation of the real SBS prevalence, as they do not reflect SBS patients who either did not require PN or were able to be successfully weaned from PN. As reasons for the differences between the US and Europe, they quote that the rate of HPN in the US might be higher, as patients may be discharged from the hospital sooner on home PN due to financial considerations, as well as that the organization of HPN resources in the US is well established and available [12][13].
2. Clinical Implications of Short Bowel Syndrome
2. Clinical Implications of SBS
SBS is associated with significant fluid and electrolyte losses and decreased ability to absorb macronutrients (i.e., carbohydrates, fat and protein), vitamins and minerals [16][17]. Clinical symptoms of SBS include diarrhea, steatorrhea, malnutrition, dehydration and, ultimately, failure to thrive [1][2]. In the past, SBS was associated with a high mortality rate. Advances in medical care, including the use of PN, has improved survival and quality of life, and generally improved the prognosis of children affected by SBS in developed countries. The goal of treatment is to increase the absorption capacity of the remaining bowel in order to achieve enteral autonomy [1][2]. Enteral autonomy is defined by the American Society for Parenteral and Enteral Nutrition (ASPEN) as “the maintenance of normal growth and hydration status by means of enteral support without the use of parenteral support for a period of more than three consecutive months” [17][18].
2.1. Proximal Versus Distal Small Bowel Loss
Patients affected by proximal resections (i.e., jejunum) tend to have a transitory but significant hypersecretion of gastric acid lasting a few weeks to months. This is likely due to unopposed gastric acid secretion by decreased production of endogenous inhibitors such as secretin, cholecystokinin, neurotensin and vasoactive intestinal peptide. Gastric hypersecretion results in inactivation of pancreatic enzymes, aggravation of diarrhea, precipitation of peptic ulcer disease and worsened malabsorption [18][19][19,20]. Nonetheless, patients with proximal resections seem to have better postoperative bowel function than those with distal resections (i.e., ileum). The ileum has significant fluid and electrolyte absorptive capacity and is the main site of bile acid and vitamin B12 absorption [16][20][17,21]. Additionally, reduced production of intestinal mediators peptide YY (PYY) and glucagon-like-peptide 1 (GLP-1), which all are produced in the ileum and colon and physiologically inhibit intestinal motility, results in increased intestinal transit time. Glucagon-like-peptide 2 (GLP-2) is also produced in the ileum and the colon and increases nutrient absorption, maintains mucosal integrity, and enhances small and large intestinal villus and crypt cell growth; therefore, these beneficial effects are diminished in distal resections [18][19].
Expression of nutrient (i.e., amino acid, peptide and monosaccharide) transporters along the small intestine is asymmetric, with some transporters being expressed to a greater extent in the jejunum and others in the ileum [21][22][23][24][22,23,24,25]. Furthermore, transporter expression does not necessarily reflect absorption. While some transporters have been shown to functionally interact [25][26][27][28][26,27,28,29], others depend on the presence of certain accessory proteins (e.g., angiotensin converting enzyme 2 (ACE2)) [21][24][29][22,25,30]. Expression of some transport proteins such as GLUT2 and SGLT1 is comparable between the newborn and adult small intestine. This extends to the expression of mRNA of the transporters GLUT1, GLUT2, GLUT7 and SGLT1. GLUT5, on the other hand, is not expressed in the newborn human gut on a protein level, but its mRNA is expressed similarly as in adults [23][24].
2.2. Choleretic Diarrhea
Increased amounts of bile acids entering the colon result in elevated bacterial deconjugation to free bile acids and subsequent stimulation of chloride and water secretion, as well as intestinal motility, a condition referred to as choleretic diarrhea [19][20]. A disrupted enterohepatic circulation, which manifests as diminished bile acid reabsorption and subsequent supersaturation of cholesterol in bile, alone or in conjunction with bile stasis due to little to no enteral stimulation in patients on PN, may lead to cholelithiasis. This condition occurs in 25–45% of SBS patients [18][19].
2.3. Small Intestinal Bacterial Overgrowth
Another significant issue in SBS is small intestinal bacterial overgrowth (SIBO). Following massive resection, the remaining bowel undergoes intestinal adaptation in order to increase fluid and nutrient absorption to compensate for lost bowel. This manifests as structural and functional changes, i.e., increase in absorptive capacity due to bowel dilatation and decrease in bowel transit time [30][31]. Furthermore, enterocytic hyperplasia, increased villous height and crypt depth, and increased expression of transporter proteins occur [31][32]. This process starts shortly after surgery and continues for up to 60 months, being most active in the first two years [32][1]. Both altered intestinal motility and bacterial translocation, as occurs in cases of ileocecal valve (ICV) resection, act as risk factors for SIBO [16][17]. Additionally, some medications such as antimotility agents or acid-suppressing drugs have the potential to disrupt native physiological flora and stimulate SIBO [19][20]. SIBO may result in mucosal inflammation, either due to direct cytopathic effects of bacteria or inappropriate immune reactions to absorbed bacterial antigens [30][31]. The consequences of mucosal inflammation may manifest as epithelial changes; for example, blunting of villi [33], less visible damage to the brush border membrane and/or production of inflammatory mediators/cytokines, all of which impair absorption [30][31]. Intraluminal bacterial degradation of carbohydrates leads to production of carbon dioxide, methane or hydrogen. Intraluminal deconjugation of bile acids results in insufficient concentrations, leading to fat malabsorption [30][31]. In this setting, the clinical symptoms of SIBO include gas, bloating, abdominal discomfort, diarrhea, steatorrhea, oxalate kidney stones and symptoms of deficiencies of fat-soluble vitamins A, D and E, but rarely vitamin K, as enteric bacteria are able to produce some amounts of vitamin K and folate [30][31]. Impaired vitamin B12 absorption, due to bacterial sequestration, may lead to macrocytic anemia and neurologic symptoms [30][31]. Intestinal inflammation can also manifest in anastomotic ulcerations resulting in chronic bleeding and microcytic anemia [30][31]. Another rare complication in children with SBS is D-lactic acidosis, which occurs due to degradation of excess carbohydrates by lactobacillus bacteria producing D-lactic acid. Symptoms include acidosis, lethargy and confusion [16][17]. In addition to the aforementioned symptoms and conditions, other potential complications of SIBO include recurring bacterial infections and sepsis, failure to thrive and delayed weaning from PN [30][31]. Duodenal aspirate and culture are considered the gold standard in the diagnosis of SIBO, with cut-offs defined as >103 colony forming units (CFU) coliforms/mL [34]. However, this technique has several limitations, as it is costly and invasive due to the requirement of upper endoscopy. In addition, it is prone to contamination by upper gastrointestinal flora which may yield false-positive results, and since mid and distal segments of the small intestine are beyond the reach of regular endoscopes, cultures from duodenal aspirates may be false-negative [35][36][35,36]. Another possible way of diagnosing SIBO is measurement of exhaled hydrogen upon ingestion of either 75 g of glucose or 10 g of lactulose. A rise in exhaled hydrogen of at least 20 parts per million (ppm) compared to baseline 90 min after oral ingestion is diagnostic for SIBO [34]. Although breath tests are used more frequently, they lack universal acceptance [35]. A recent comparative study by Cangemi et al. comparing duodenal aspirate (DA) to the lactulose breath test (LBT) showed that contamination is frequent in DA (19.8%) and there is poor result agreement between DA and LBT (63.5%). Therefore, the authors recommend the use of LBT as it is cheaper, safer, and less likely to yield a contaminant result [37].
3. Conservative Treatment
The ultimate goal of SBS treatment is to achieve full intestinal autonomy and to reduce long-term dependence on parenteral support by increasing the absorptive capacity of the remnant bowel [16][17]. Current therapeutic approaches follow a sequential strategy. The first-line therapy consists of parenteral nutrition, promotion of enteral feeding, restoration of bowel continuity, closure of enterostomies as early as possible, the use of dietary supplementations and, where indicated, antibiotics [38]. In some cases, intestinal growth can be stimulated by the use of growth factors [38].
3.1. Parenteral Nutrition
Since its advent in the late 1960s [39], PN represents a cornerstone in the conservative treatment of SBS patients. PN aims to replete calories and nutrients while bypassing the enteral circuit [16][17]. The European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), European Society for Clinical Nutrition and Metabolism (ESPEN), European Society of Paediatric Research (ESPR) and Chinese Society of Parenteral and Enteral Nutrition (CSPEN) provides recommendations in its 2018 published guideline on the use of pediatric parenteral nutrition [40]. The guideline recommends the use of Schofield’s equation for calculation of resting energy expenditure (REE), which is based on age, weight and gender; additionally, energy requirements for preterm and term newborns and infants for PN in different stages of disease are provided [41]. Exact parenteral glucose supply recommendations for preterm and term newborns, as well as infants and children, are provided, the latter according to their body weight and phase of illness. Hyperglycemia (>145 mg/dL) should be avoided in pediatric and neonatal ICU patients due to increased morbidity and mortality. In addition, repetitive and/or prolonged hypoglycemia (<45 mg/dL) should be avoided in all ICU patients. Excessive glucose intake should be avoided, as hyperglycemia causes liver steatosis and enhanced hepatic VLDL production [42]. Parenteral amino acid intake in preterm newborns should be at least 1.5 g/kg/d on the first postnatal day and between 2.5 g/kg/d and 3.5 g/kg/d from postnatal day two onwards, alongside non-protein intakes >65 kcal/kg/d and adequate micronutrient intake. Term infants should receive between 1.5 g/kg/d and 3.0 g/kg/d of amino acids, and children and adolescents between 1.0 g/kg/d and 2.0 g/kg/d [43]. The first choice of treatment should be 20% intravenous lipid emulsions (ILEs). Parenteral lipid intake should be limited to 4 g/kg/d in preterm and term infants, respectively, and 3 g/kg/d in children, while markers of liver integrity and function, alongside triglyceride concentrations, should be monitored regularly in patients receiving ILEs. Consideration should be given to reducing ILE dosage if serum or plasma triglyceride concentration exceeds 265 mg/dL in infants or 400 mg/dL in older children [44]. Infants and children on PN should receive parenteral vitamins daily, except for vitamin K which should be given weekly [45]. Newborns and children requiring prolonged PN during hospitalization should receive either a peripherally inserted central catheter (PICC) or a tunneled central vein catheter (CVC); for long-term or home PN children a tunneled CVC is the preferred choice. Where possible, a CVC should only be used for administering PN. Taurolidine is effective in preventing catheter related bloodstream infections (CRBSI) and should be used during long-term catheter use. Use of heparin flush over saline flush to prevent thrombotic occlusion has no proven benefit in children and is, therefore, not recommended [46]. Standard PN solutions, over individualized PN solutions, should be used in the majority of pediatric and newborn patients including very-low-birthweight premature infants [47].
The ESPGHAN/ESPEN/ESPR/CSPEN guidelines also provide recommendations on complications [48], fluid and electrolytes [49], iron and trace minerals [50], calcium, phosphorus and magnesium [51], home parenteral nutrition [52] and organizational aspects [53] in PN. A study by Gonzalez-Hernandez et al. on clinical variables predicting time to weaning from PN showed that time to full enteral nutrition is strongly associated with both the remaining small bowel length and the percentage of expected remaining small bowel length based on gestational age (GA). Children who have 26–50% of expected small bowel remaining required PN for approximately two years, compared to children with 51–75% remaining of expected small bowel who required PN for approximately one year. Factors that showed no difference in time to full enteral nutrition were gender, birth weight, GA, underlying diagnosis requiring intestinal resection, presence or absence of the ICV and type of anastomosis (small bowel to small bowel anastomosis vs. small bowel to colon anastomosis) [54].
3.2. Medical Management
The focus of medical treatment is to compensate for fluid, electrolyte and nutrient losses, to limit diarrhea and promote adequate weight gain and growth [16][17].
3.2.1. Antimotility Agents
The first-line therapy for diarrhea is antimotility agents such as loperamide or diphenoxylate-atropine [19][20]. Loperamide is a peripherally acting µ-opioid receptor agonist and is not associated with undesirable central nervous system (CNS) effects, such as sedation or addiction [19][20]. In contrast to loperamide, the opioid receptor agonist diphenoxylate crosses the blood–brain barrier [55]. Nevertheless, the abuse potential of diphenoxylate is limited, as atropine causes anticholinergic side effects such as xerostomia, tachycardia or mydriasis when taken in high doses [55]. Other second- or third-line treatments are opioids such as codeine, morphine or opium tincture, which are not restricted to the peripheral nervous system and can induce significant CNS effects [56]. Unlike tolerance to analgesic effects, tolerance to antidiarrheal effects of opioids and opioid-receptor agonists are rare [19][57][20,57].
3.2.2. Choleretic Diarrhea Treatment
Patients with choleretic diarrhea can be treated with bile-acid binding resins such as cholestyramine, colestipol or colesevelam. These are non-digestible anion exchange polymers that bind to bile acids in the colon and form insoluble complexes, which are then excreted [19][20]. In patients with extensive ileal resection, bile-acid binding resins may not be appropriate, because the loss of bile acids might be greater than the hepatic synthesis, and treatment with resins may aggravate fat malabsorption and steatorrhea [19][20]. Furthermore, resins should be used with caution because of possible interactions (i.e., binding and reducing activity) with other drugs such as loperamide or nonsteroidal anti-inflammatory drugs [19][20].
3.2.3. Inhibition of Gastric Hypersecretion
Gastric hypersecretion can be addressed with several drug classes. Proton pump inhibitors (PPI) such as pantoprazole, omeprazole, lansoprazole or esomeprazole, which irreversibly block the H+-K+-ATPase proton pump, are the first-line treatment and show great efficacy [19][20]. Although generally well tolerated, long-term use of PPIs increases the risk for osteoporosis, bone fractures and vitamin B12 deficiency [58][59][58,59]. Upon discontinuation, rebound hypersecretion may occur [60]. As gastric acid physiologically reduces the concentration of ingested bacteria, PPI treatment may stimulate SIBO [61]. Histamine type 2 (H2) receptor antagonists (e.g., famotidine, ranitidine or cimetidine) block the action of the acid secretion mediator histamine, which is released from gastric mucosa upon stimulation by gastrin. They act as second-line agents along with a2-adrenergic receptor agonists, such as clonidine, although evidence for use of the latter is scant [19][20]. Third-line treatment includes the lone-acting somatostatin analogue octreotide, which inhibits gastrin and other GI hormones [19][20]. The use of octreotide has several limitations, including high cost, inconvenient administration (subcutaneous injection) and possible risk of adverse effects such as cholelithiasis [19][20].
3.2.4. Treatment of Small Intestinal Bacterial Overgrowth
The American College of Gastroenterologists (ACG) recommends antibiotic treatment for SIBO in order to eradicate overgrowth and resolve symptoms, citing a meta-analysis of 24 cohort studies, 7 randomized controlled trials (RCTs) and 1 randomized crossover study showing rifaximin is effective and safe for treating SIBO [34][62][34,62]. Other antibiotics such as amoxicillin-clavulanic acid, ciprofloxacin, norfloxacin, doxycycline, metronidazole, neomycin, tetracycline and trimethoprim-sulfamethoxazole are also suggested in the ACG guideline, with variable efficacies in eradicating SIBO ranging from 30% to 100%; in general, evidence is limited to small clinical studies of poor to modest quality [34]. A meta-analysis of twelve RCTs, two crossover trials, four prospective single-arm trials and four retrospective studies on the use of probiotics in SIBO showed that probiotics are effective in terms of SIBO decontamination, reduction in H2 concentration and abdominal pain relief, but not in prevention of SIBO. The majority of included studies (21/22) focused on adult patients [63]. Furthermore, a systematic review of one crossover RCT, one case control study and nine case reports on the use of probiotics in children with SBS showed insufficient evidence [64].
3.2.5. Glucagon-Like Peptide-2
A subcutaneously administered glucagon-like peptide-2 (GLP-2) analogue (teduglutide) has been found to increase intestinal absorption and reduce the need for parenteral support. It is approved in the European Union for SBS patients older than one year who are stable after a period of postoperative intestinal adaptation. Dosage is 0.05 mg/kg/day for both children and adults, and contraindications are active or suspected malignancy and/or a recent (i.e., ≤5 years) history of gastrointestinal (GI) or hepatobiliary malignancy. Teduglutide is generally well tolerated, but the most common adverse effects are abdominal distention and GI stoma complications (swelling of the stoma, etc.), each occurring in approximately 20% of patients [65]. One of the most important studies on the efficacy and safety of teduglutide in pediatric patients with intestinal failure due to SBS was a 24-week phase III study by Kocoshis et al.; 59 patients aged 1–17 years were randomized into either the 0.025 mg/kg/d (n = 24) or the 0.05 mg/kg/d (n = 26) or the standard of care (SOC) group (n = 9). Primary endpoint was a ≥20% reduction in parenteral support from baseline in week 24, which was achieved by 54.2% (n = 13) in the 0.025 mg/kg/d group, 69.2% (n = 18) in the 0.05 mg/kg/d group and 11.1% (n = 1) in the SOC group (p < 0.05 vs. SOC). In total, five patients, two receiving 0.025 mg/kg/d (8%) and three receiving 0.05 mg/kg/d (12%), but no patient in the SOC group, achieved enteral autonomy [66].
3.2.6. Pancreatic Enzyme Replacement and Bile Acid Supplementation
Fat malabsorption can be treated with pancreatic enzyme replacement (i.e., pancrelipase). In order to maximize effectiveness, therapy should only be started following the normalization of gastric hypersecretion and GI motility with acid secretion and antimotility drugs. Bile acid supplementation may be beneficial to patients who cannot synthesize enough to compensate for losses. However, this should be used with caution, as exacerbation of diarrhea constitutes a possible side effect. Both pancreatic enzymes and bile acids should be taken with meals [19][20]. However, there are no data available on the use of bile acid supplementation in children with SBS.
3.2.7. Chyme Reinfusion
An additional possible conservative treatment for intestinal failure in cases where patients have double enterostomies or entero-atmospheric fistulas is chyme reinfusion (CR). Here, upstream chyme is sucked and pumped into a tube inserted into the downstream intestine by a peristaltic pump in order to artificially restore bowel continuity pending surgical closure [67]. It is recommended by the American Society for Parenteral and Enteral Nutrition (ASPEN) [68] and the ESPEN [69][70][71][69,70,71] whenever possible. CR is very effective in restoring intestinal function, reducing the need for intravenous support by 89% [67]. CR is also effective in neonatal and pediatric populations, but available data are limited [72]. A study by Gause et al. on mucous fistula refeeding in preterm neonates after bowel resection and small bowel enterostomy demonstrated that, in the inter-operative period, CR patients reached goal enteral feedings and were able to have PN discontinued significantly earlier than patients with an enterostomy only, without creation of a mucous fistula and refeeding efforts. Moreover, after anastomosis, refeeding patients reached goal enteral feeds and had PN discontinued significantly earlier [73].
