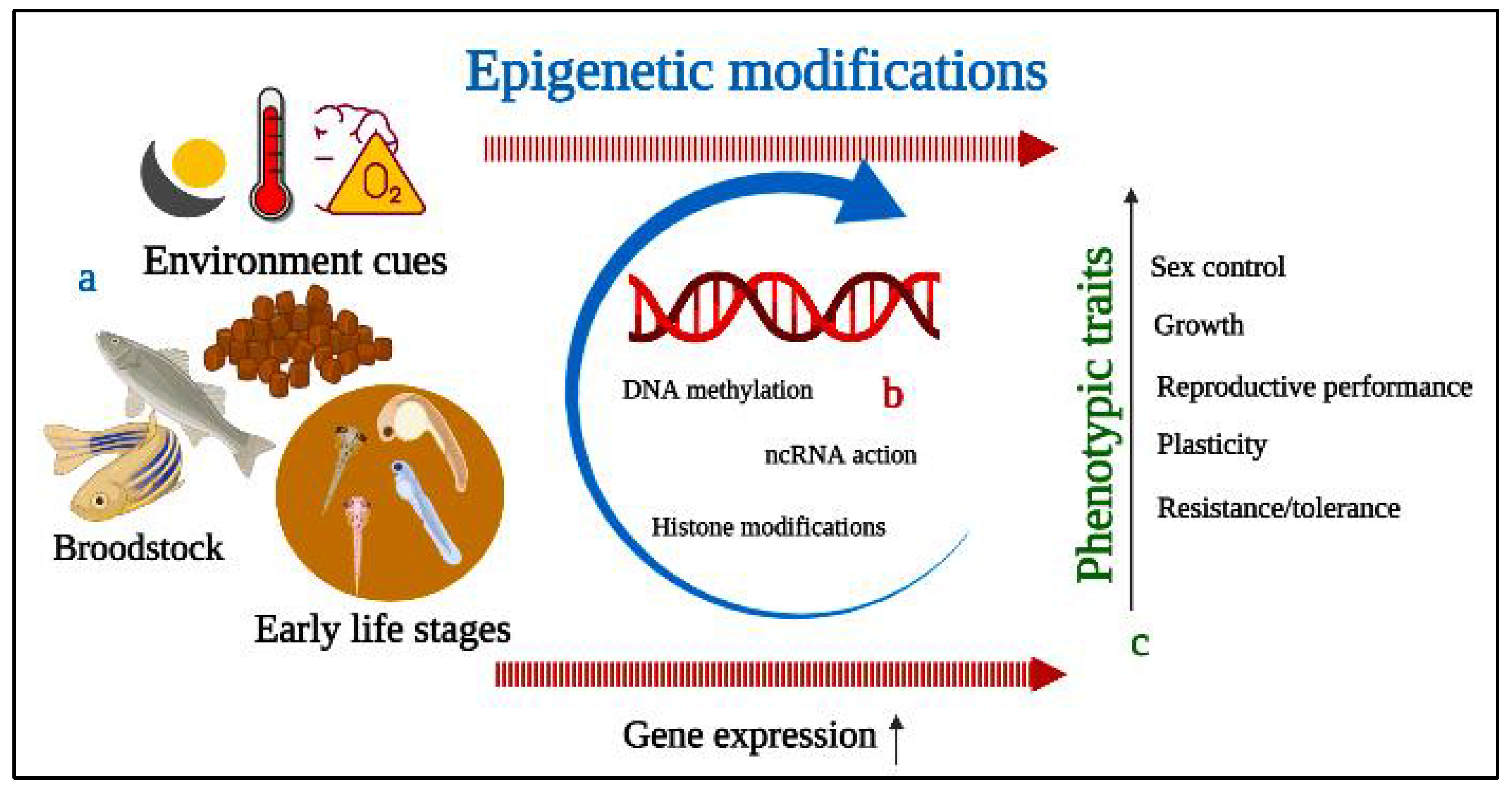
Fish represent an excellent source of animal protein as well as a biomedical research model as a result of their evolutionary relatedness and similarity with the human genome. Commercial and ornamental fish culture has achieved popularity, but reproductive dysfunctions act as a limiting factor for quality fry production, interfering with the sustainability of the aquaculture industry. Fish reproduction is crucial for any species’ existence, and reproductive performance can potentially be improved through applications of epigenetics and probiotics. Epigenetics is a highly sensitive molecular approach that includes chromatin structure and function alteration, DNA methylation, and modification of non-coding RNA molecules for the transfer of desired information from parents to offspring. DNA methyltransferase improves reproductive cyp11a1, esr2b, and figla gene expression and feminizes zebrafish (Danio rerio). Moreover, epigenetics also contributes to genome stability, environmental plasticity, and embryonic development. However, methylation of specific genes can negatively affect sperm quality, resulting in poor fertilization. Probiotic administration is able to induce responsiveness of incompetent follicles to maturation-inducing hormones and can change oocyte chemical composition during vitellogenic development. The positive role of probiotics on testicular cells is validated by upregulating the transcription levels of leptin, bdnf, and dmrt1 genes facilitating the spermatogenesis.
- epigenetics
- probiotics
- reproductive dysfunctions
- gene transcription
- ornamental fish
- commercial fish
1. Introduction
2. Fish Reproductive Dysfunctions
Globally, aquaculture is continually striving for the consistency of physiological integrity of fingerlings through standardized reproductive programmes, which are a key objective for sustainable aquaculture [45]. Brood fish rearing and their management strategies have been considered as vital for aquaculture production for the last three decades. Generally, many species of captive fishes do not perform normal reproduction (especially females), a phenomenon potentially caused by a lack of natural spawning stimuli, specifically failure of oocyte maturation [46,47][46][47]. Supplied protein, fatty acids, lipid, vitamins, especially E and C, and carotenoids also influence fish Fec, FR, HR, and larval development [48,49][48][49]. Moreover, presence of chemical fertilizers, antibiotics, hormones and industrial effluents, environmental degradation, and frequent fluctuation of temperature in nature and captivity suppress the immunity and beneficial microbial activity in broodstock fishes, making them more susceptible to infectious disease [27,50,51,52][27][50][51][52]. Hypothalamus, pituitary, and gonads form the hypothalamic-pituitary-gonadal (HPG) axis, which regulates reproductive function in most fishes [53]. The hypothalamus and the pituitary glands modulate the production of pituitary gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH), and gonadal sex steroids (SS) regulate vitellogenesis and oocyte maturation. FSH and LH have been confirmed to figure importantly in oocyte growth and maturation [54]. Gioacchini et al. [7] have categorized three types of female broodstocks reproductive dysfunctions based on the affected pattern of reproductive cycle whereas, Selvaraj, et al. [55] documented two modes of dysfunction of teleost fishes (Figure 1). Both groups of investigators associated the most disruptive reproductive dysfunctions occurred with the vitellogenic phase, as exemplified by failed vitellogenesis in Anguilla spp. and Seriola spp. [56] and Mugil cephalus (Grey Mullet) [57]. The second mode is categorized as the inability to reach final oocyte maturation to ovulation as seen in farmed white and striped bass [58,59,60][58][59][60] and in Cyprinidae [61]. The third set of dysfunctions leads to failures to spawn in the breeding season; sometimes E. aeneus, M. saxatilis, and Dentex dentex (common dentex) females may release eggs after ovulations without exhibiting characteristic breeding behavior [7,62][7][62]. Under some circumstances, cultured salmonids fail to complete the reproductive cycle and eggs in the abdominal cavity are reabsorbed over the following months [63]. These are examples of failures among female broodstocks that do not appear to be accompanied by reproductive problems that can be attributed to male fishes. One common problem faced by cultured male fishes is low quantity or quality of milt production during spermiation [60]. The presence of both male and female germ cells in the testis tissue is referred to as an intersex state caused by endocrine disruption through chemical exposure and hormonal imbalance [64]. The histopathological assessment of gonadal tissue during the development stage allows the detection of intersex reproductive dysfunction in male Japanese medaka (Oryzias latipes) [65].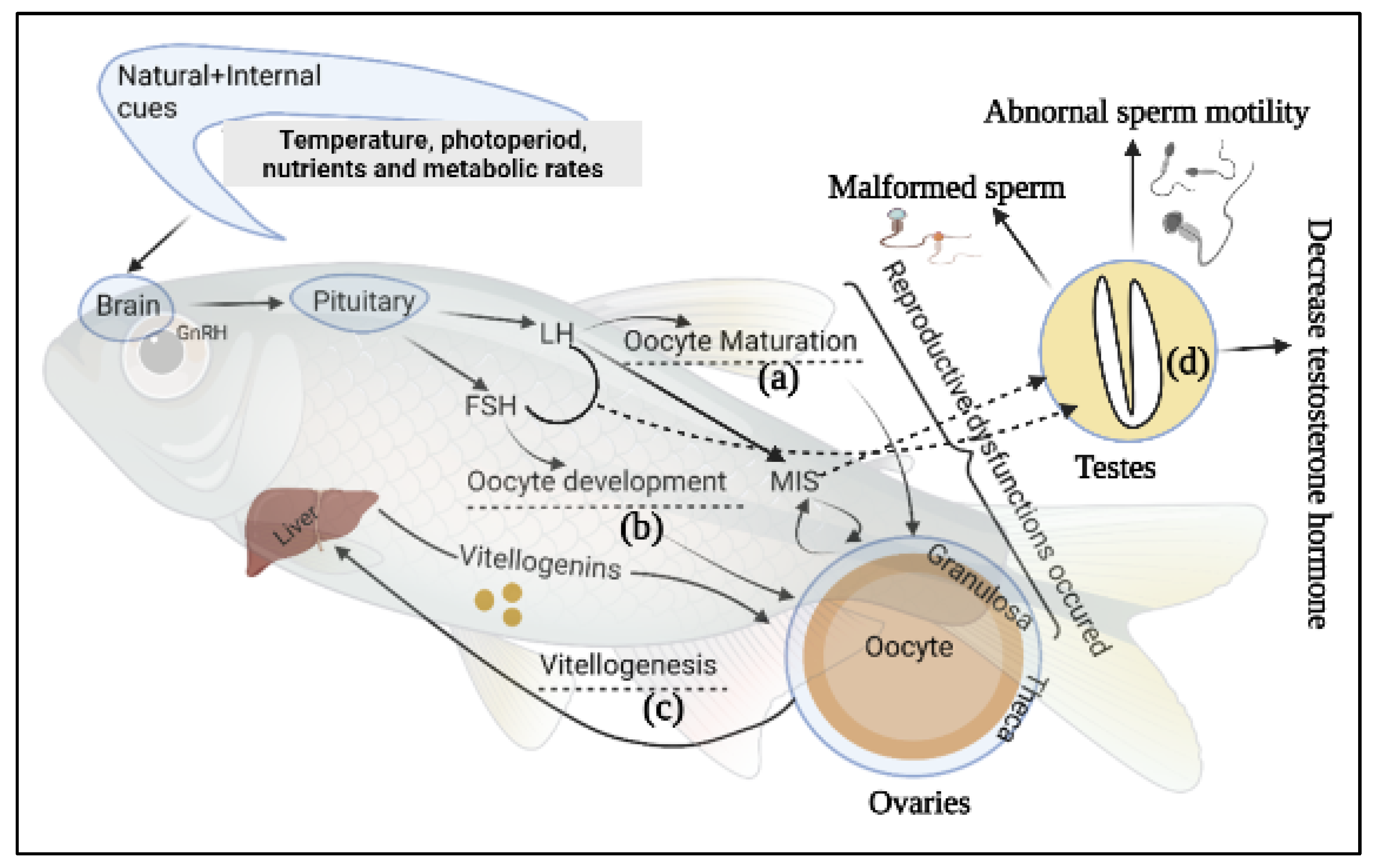
3. Epigenetics Mechanism and Modifications of Fish Reproductive Performance
4. Influence of Probiotics on Reproductive Performance of Fish
4.1. Ornamental Fish
4.1.1. Male
4.1.2. Female
| Supplemented Probiotics | Fish Species | Fish Number | Duration | Concentration | Effects on Fish | References |
|---|---|---|---|---|---|---|
| Bacillus subtilis | Poecilia reticulata (Guppy), P.sphenops (Valenciennes), Xiphophorus helleri (Swordtail fish) and X. maculatus (Platyfish) | 60 virgin females of each species | 365 days | 5 × 107–5 × 108 CFU g−1 and 5 × 105–5 × 106 CFU g−1 | EP Fec and GSI ↑; SR (fry) ↑; Fry death and deformities ↓ | [27] |
| Lactobacillus rhamnosus IMC 501 | Danio rerio (Zebrafish) | 10 females | 10 days | 106 CFU g−1 | EP Fec, GSI, and Ovolution rate ↑; Oocyte maturation G and FD ↑; | [29,31,176,185][29][31][107][117] |
| Oocyte maturation FD and FM ↑ | [170][100] | |||||
| Follicular survival ↑ and apoptosis ↓ | [30] | |||||
| Lab. rhamnosus IMC 501 | D. rerio | 40 males and females | 10 days | 106 CFU g−1 | Embryo development ↑; HR ↑ | [134][69] |
| Pediococcus acidilactici (Bactocell®) | D. rerio | 5 wild males | 10 days | 106 CFU g−1 | SP testicular cells ↑ | [164][118] |
| Lab. rhamnosus CECT8361 and Bifidobacterium longum CECT7347 | D. rerio | 36 Males | 21 days | 109 CFU g−1 | SP SQ, SDn, SM ↑ | [182][114] |
| PrimaLac® (Lab. acidophilus, Lab. casei, Enterococcus faecium, Bifidobacterium thermophilum) | X. helleri | 10 females and 3 males | 182 days | 0.04%, 0.09% and 0.14% | EP Fec and GSI ↑; SR (fry) ↑ | [28] |
| P. acidilactici | Carassius auratus (Goldfish) | 720 fishes | 180 days | 0.1, 0.2, and 0.3% | EP GSI, HSI, AF, RF, ED, OD, FR, and HR ↑; SP SM, SD, SDn, and Stc ↑ | [133][68] |
| Lab. rhamnosus IMC 501 | Fundulus heteroclitus (Killifish) | 10 females and 10 males | 8 days | 106 CFU mL−1 | EP GSI, Fec ↑ and HR →; SR (fry) ↑; GP L and W ↑ | [36] |
References
- Liao, I.C.; Chao, N.-H. Aquaculture and food crisis: Opportunities and constraints. Asia Pac. J. Clin. Nutr. 2009, 18, 564–569.
- Merrifield, D.L.; Ringo, E. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2014.
- Akhtar, M.; Ciji, A.; Sarma, D.; Rajesh, M.; Kamalam, B.; Sharma, P.; Singh, A. Reproductive dysfunction in females of endangered golden mahseer (Tor putitora) in captivity. Anim. Reprod. Sci. 2017, 182, 95–103.
- Mañanós, E.; Duncan, N.; Mylonas, C. Reproduction and control of ovulation, spermiation and spawning in cultured fish. In Methods in Reproductive Aquaculture: Marine and Freshwater Species; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2008; pp. 3–80.
- Ottolenghi, F.; Silvestri, C.; Giordano, P.; Lovatelli, A.; New, M.B. Capture-Based Aquaculture: The Fattening of Eels, Groupers, Tunas and Yellowtails; FAO: Mexico City, Mexico, 2004.
- Guzmán, J.; Luckenbach, A.; Goetz, F.W.; Fairgrieve, W.T.; Middleton, M.A.; Swanson, P. Reproductive dysfunction in cultured sablefish (Anoplopoma fimbria). Bull. Fish. Res. Agency 2015, 40, 111–119.
- Gioacchini, G.; Giorgini, E.; Vaccari, L.; Carnevali, O. Can Probiotics Affect Reproductive Processes of Aquatic Animals? In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 328–346.
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of probiotics in aquaculture. Int. Sch. Res. Not. 2012, 2012, 916845.
- Granada, L.; Lemos, M.F.; Cabral, H.N.; Bossier, P.; Novais, S.C. Epigenetics in aquaculture—The last frontier. Rev. Aquac. 2018, 10, 994–1013.
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes Dev. 2009, 23, 781–783.
- Herráez, M.P.; Lombó, M.; González-Rojo, S. The Role of Epigenetics in Fish Biology and Reproduction: An Insight into the Methods Applied to Aquaculture. In Cellular and Molecular Approaches in Fish Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 69–104.
- Gavery, M.R.; Roberts, S.B. Epigenetic considerations in aquaculture. PeerJ. 2017, 5, e4147.
- Hanson, M.A.; Skinner, M.K. Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenet. 2016, 2, dvw002.
- Kelley, J.L.; Tobler, M.; Beck, D.; Sadler-Riggleman, I.; Quackenbush, C.R.; Rodriguez, L.A.; Skinner, M.K. Epigenetic inheritance of DNA methylation changes in fish living in hydrogen sulfide—Rich springs. Proc. Natl. Acad. Sci. USA 2021, 118, e2014929118.
- González-Recio, O. Epigenetics: A new challenge in the post-genomic era of livestock. Front. Genet. 2012, 2, 106.
- Woods, L.C., III; Li, Y.; Ding, Y.; Liu, J.; Reading, B.J.; Fuller, S.A.; Song, J. DNA methylation profiles correlated to striped bass sperm fertility. BMC Genom. 2018, 19, 244.
- Renn, S.C.; Hurd, P.L. Epigenetic regulation and environmental sex determination in cichlid fishes. Sex. Dev. 2021, 15, 93–107.
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.-N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260.
- Shao, C.; Li, Q.; Chen, S.; Zhang, P.; Lian, J.; Hu, Q.; Sun, B.; Jin, L.; Liu, S.; Wang, Z.; et al. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 2014, 24, 604–615.
- Labbé, C.; Robles, V.; Herraez, M.P. Epigenetics in fish gametes and early embryo. Aquaculture 2017, 472, 93–106.
- Hasan, K.N.; Banerjee, G. Recent studies on probiotics as beneficial mediator in aquaculture: A review. J. Basic Appl. Zool. 2020, 81, 53.
- Hasan, M.T.; Jang, W.J.; Kim, H.; Lee, B.-J.; Kim, K.W.; Hur, S.W.; Lim, S.G.; Bai, S.C.; Kong, I.-S. Synergistic effects of dietary Bacillus sp. SJ-10 plus β-glucooligosaccharides as a synbiotic on growth performance, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 82, 544–553.
- Jamal, M.T.; Sumon, A.A.; Pugazhendi, A.; Al Harbi, M.; Hussain, A.; Haque, F. Use of probiotics in commercially important finfish aquaculture. Int. J. Probiotics Prebiotics 2020, 15, 7–21.
- Hasan, M.T.; Jang, W.J.; Lee, B.-J.; Hur, S.W.; Lim, S.G.; Kim, K.W.; Han, H.-S.; Lee, E.-W.; Bai, S.C.; Kong, I.-S. Dietary Supplementation of Bacillus sp. SJ-10 and Lactobacillus plantarum KCCM 11322 Combinations enhance growth and cellular and humoral immunity in olive flounder (Paralichthys olivaceus). Probiotics Antimicrob. Proteins 2021, 13, 1277–1291.
- Hasan, M.T.; Je Jang, W.; Lee, J.M.; Lee, B.-J.; Hur, S.W.; Gu Lim, S.; Kim, K.W.; Han, H.-S.; Kong, I.-S. Effects of immunostimulants, prebiotics, probiotics, synbiotics, and potentially immunoreactive feed additives on olive flounder (Paralichthys olivaceus): A review. Rev. Fish. Sci. Aquac. 2019, 27, 417–437.
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. In Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO Food and Nutrition Paper—Series Number: 0254-4725|1014-2908; FAO/WHO: Cordoba, Argentina, 2001; p. 56.
- Ghosh, S.; Sinha, A.; Sahu, C. Effect of probiotic on reproductive performance in female livebearing ornamental fish. Aquac. Res. 2007, 38, 518–526.
- Abasali, H.; Mohamad, S. Effect of dietary supplementation with probiotic on reproductive performance of female livebearing ornamental fish. Res. J. Anim. Sci. 2010, 4, 103–107.
- Gioacchini, G.; Bizzaro, D.; Giorgini, E.; Ferraris, P.; Sabbatini, S.; Carnevali, O. P-230 Oocytes maturation induction by Lactobacillus rhamnosus in Danio rerio: In vivo and in vitro studies. Hum. Reprod. 2010, 25, I205–I206.
- Gioacchini, G.; Dalla Valle, L.; Benato, F.; Fimia, G.M.; Nardacci, R.; Ciccosanti, F.; Piacentini, M.; Borini, A.; Carnevali, O. Interplay between autophagy and apoptosis in the development of Danio rerio follicles and the effects of a probiotic. Reprod. Fertil. Dev. 2013, 25, 1115–1125.
- Gioacchini, G.; Giorgini, E.; Ferraris, P.; Tosi, G.; Bizzaro, D.; Silvi, S. Could probiotics improve fecundity? Danio rerio as case of study. J. Biotechnol. 2010, 150, 59–60.
- Giorgini, E.; Conti, C.; Ferraris, P.; Sabbatini, S.; Tosi, G.; Rubini, C.; Vaccari, L.; Gioacchini, G.; Carnevali, O. Effects of Lactobacillus rhamnosus on zebrafish oocyte maturation: An FTIR imaging and biochemical analysis. Anal. Bioanal. Chem. 2010, 398, 3063–3072.
- Miccoli, A.; Gioacchini, G.; Maradonna, F.; Benato, F.; Skobo, T.; Carnevali, O. Beneficial bacteria affect Danio rerio development by the modulation of maternal factors involved in autophagic, apoptotic and dorsalizing processes. Cell. Physiol. Biochem. 2015, 35, 1706–1718.
- Valcarce, D.G.; Pardo, M.; Riesco, M.; Cruz, Z.; Robles, V. Effect of diet supplementation with a commercial probiotic containing Pediococcus acidilactici (Lindner, 1887) on the expression of five quality markers in zebrafish (Danio rerio (Hamilton, 1822)) testis. J. Appl. Ichthyol. 2015, 31, 18–21.
- Mehdinejad, N.; Imanpour, M.R.; Jafari, V. Combined or individual effects of dietary probiotic, Pediococcus acidilactici and nucleotide on reproductive performance in goldfish (Carassius auratus). Probiotics Antimicrob. Proteins 2019, 11, 233–238.
- Lombardo, F.; Gioacchini, G.; Carnevali, O. Probiotic-based nutritional effects on killifish reproduction. Fish. Aquacult J. FAJ-33 2011, 2011, FAJ-33.
- Dias, D.d.C.; Furlaneto, F.d.P.B.; Sussel, F.R.; Tachibana, L.; Gonçalves, G.S.; Ishikawa, C.M.; Natori, M.M.; Ranzani-Paiva, M.J.T. Economic feasibility of probiotic use in the diet of Nile tilapia, Oreochromis niloticus, during the reproductive period. Acta Scientiarum. Anim. Sci. 2020, 42, e47960.
- Akbari Nargesi, E.; Falahatkar, B.; Sajjadi, M.M. Dietary supplementation of probiotics and influence on feed efficiency, growth parameters and reproductive performance in female rainbow trout (Oncorhynchus mykiss) broodstock. Aquac. Nutr. 2020, 26, 98–108.
- Rahman, M.L.; Akhter, S.; Mallik, M.K.M.; Rashid, I. Probiotic enrich dietary effect on the reproduction of butter catfish, Ompok pabda (Hamilton, 1872). Int. J. Curr. Res. Life Sci. 2018, 7, 866–873.
- Ariole, C.N.; Okpokwasili, G.C. The effect of indigenous probiotics on egg hatchability and larval viability of Clarias gariepinus. Rev. Ambiente Água 2012, 7, 81–88.
- Vílchez, M.C.; Santangeli, S.; Maradonna, F.; Gioacchini, G.; Verdenelli, C.; Gallego, V.; Peñaranda, D.S.; Tveiten, H.; Pérez, L.; Carnevali, O.; et al. Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis. Theriogenology 2015, 84, 1321–1331.
- Hasan, M.T.; Jang, W.J.; Lee, S.; Kim, K.W.; Lee, B.J.; Han, H.S.; Bai, S.C.; Kong, I.S. Effect of β-glucooligosaccharides as a new prebiotic for dietary supplementation in olive flounder (Paralichthys olivaceus) aquaculture. Aquac. Res. 2018, 49, 1310–1319.
- Hasan, M.T.; Jang, W.J.; Lee, B.-J.; Kim, K.W.; Hur, S.W.; Lim, S.G.; Bai, S.C.; Kong, I.-S. Heat-killed Bacillus sp. SJ-10 probiotic acts as a growth and humoral innate immunity response enhancer in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2019, 88, 424–431.
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671.
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633.
- Mylonas, C.C.; Zohar, Y. Promoting oocyte maturation, ovulation and spawning in farmed fish. In The Fish Oocyte; Springer: Berlin/Heidelberg, Germany, 2007; pp. 437–474.
- Papadaki, M.; Peleteiro, J.B.; Alvarez-Blázquez, B.; Villanueva, J.L.R.; Linares, F.; Vilar, A.; Rial, E.P.; Lluch, N.; Fakriadis, I.; Sigelaki, I.; et al. Description of the annual reproductive cycle of wreckfish Polyprion americanus in captivity. Fishes 2018, 3, 43.
- Fernando, A.; Phang, V.; Chan, S. Diets and feeding regimes of poeciliid fishes in Singapore. Asian Fish. Sci 1991, 4, 99–107.
- Izquierdo, M.; Fernandez-Palacios, H.; Tacon, A. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42.
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573.
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378.
- Wong, S.; Rawls, J.F. Intestinal microbiota composition in fishes is influenced by host ecology and environment. Mol. Ecol. 2012, 21, 3100–3102.
- Maruska, K.P.; Fernald, R.D. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology 2011, 26, 412–423.
- Zhang, Z.; Zhu, B.; Ge, W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol. Endocrinol. 2015, 29, 76–98.
- Selvaraj, S.; Chidambaram, P.; Ezhilarasi, V.; Kumar, P.P.; Moses, T.; Antony, C.; Ahilan, B. A Review on the Reproductive Dysfunction in Farmed Finfish. Annu. Res. Rev. Biol. 2021, 36, 65–81.
- Abellan, E.; Basurco, B. Marine Finfish Species Diversification: Current Situation and Prospects in Mediterranean Aquaculture; CIHEAM-IAMZ: Zaragoza, Spain; FAO: Rome, Italy, 1999.
- Monbrison, D.D.; Tzchori, I.; Holland, M.C.; Zohar, Y.; Yaron, Z.; Elizur, A. Acceleration of gonadal development and spawning induction in the Mediterranean grey mullet, Mugil cephalus: Preliminary studies. Isr. J. Aquac. 1997, 49, 214–221.
- Mylonas, C.C.; Magnus, Y.; Klebanov, Y.; Gissis, A.; Zohar, Y. Reproductive biology and endocrine regulation of final oocyte maturation of captive white bass. J. Fish. Biol. 1997, 51, 234–250.
- Mylonas, C.C.; Woods, L., III; Zohar, Y. Cyto-histological examination of post-vitellogenesis and final oocyte maturation in captive-reared striped bass. J. Fish. Biol. 1997, 50, 34–49.
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534.
- Podhorec, P.; Kouřil, J. Hypothalamic factors (GnRH and DA) and their utilization to elimination of reproductive dysfunction in Cyprinidae fish (a review). Bull. VÚRH Vodňany 2009, 45, 10–17.
- Zohar, Y.; Mylonas, C. Endocrine manipulations of spawning in cultured fish: From hormones to genes, Reproductive Biotechnology in Finfish Aquaculture. Aquaculture 2001, 197, 99–136.
- Bromage, N.; Jones, J.; Randall, C.; Thrush, M.; Davies, B.; Springate, J.; Duston, J.; Barker, G. Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture 1992, 100, 141–166.
- Hutchinson, T.H.; Ankley, G.T.; Segner, H.; Tyler, C.R. Screening and testing for endocrine disruption in fish—Biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006, 114, 106–114.
- Grim, K.C.; Wolfe, M.; Hawkins, W.; Johnson, R.; Wolf, J. Intersex in Japanese medaka (Oryzias latipes) used as negative controls in toxicologic bioassays: A review of 54 cases from 41 studies. Environ. Toxicol. Chem. 2007, 26, 1636–1643.
- Pierron, F.; Lorioux, S.; Héroin, D.; Daffe, G.; Etcheverria, B.; Cachot, J.; Morin, B.; Dufour, S.; Gonzalez, P. Transgenerational epigenetic sex determination: Environment experienced by female fish affects offspring sex ratio. Environ. Pollut. 2021, 277, 116864.
- Li, R.; Yang, L.; Han, J.; Zou, Y.; Wang, Y.; Feng, C.; Zhou, B. Early-life exposure to tris (1, 3-dichloro-2-propyl) phosphate caused multigenerational neurodevelopmental toxicity in zebrafish via altering maternal thyroid hormones transfer and epigenetic modifications. Environ. Pollut. 2021, 285, 117471.
- Stephens, K.E.; Miaskowski, C.A.; Levine, J.D.; Pullinger, C.R.; Aouizerat, B.E. Epigenetic regulation and measurement of epigenetic changes. Biol. Res. Nurs. 2013, 15, 373–381.
- Breton-Larrivée, M.; Elder, E.; McGraw, S. DNA methylation, environmental exposures and early embryo development. Anim. Reprod. 2019, 16, 465–474.
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617.
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38.
- Brykczynska, U.; Hisano, M.; Erkek, S.; Ramos, L.; Oakeley, E.J.; Roloff, T.C.; Beisel, C.; Schübeler, D.; Stadler, M.B.; Peters, A.H. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010, 17, 679–687.
- Wu, S.-F.; Zhang, H.; Cairns, B.R. Genes for embryo development are packaged in blocks of multivalent chromatin in zebrafish sperm. Genome Res. 2011, 21, 578–589.
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118.
- Bizuayehu, T.T.; Babiak, I. MicroRNA in teleost fish. Genome Biol. Evol. 2014, 6, 1911–1937.
- Pauli, A.; Rinn, J.L.; Schier, A.F. Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 2011, 12, 136–149.
- Andreassen, R.; Worren, M.M.; Høyheim, B. Discovery and characterization of miRNA genes in Atlantic salmon (Salmo salar) by use of a deep sequencing approach. BMC Genom. 2013, 14, 482.
- Juanchich, A.; Bardou, P.; Rué, O.; Gabillard, J.-C.; Gaspin, C.; Bobe, J.; Guiguen, Y. Characterization of an extensive rainbow trout miRNA transcriptome by next generation sequencing. BMC Genom. 2016, 17, 164.
- Nilsson, E.E.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of reproductive disease. Biol Reprod 2015, 93, 145.
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109.
- Donelson, J.M.; Wong, M.; Booth, D.J.; Munday, P.L. Transgenerational plasticity of reproduction depends on rate of warming across generations. Evol. Appl. 2016, 9, 1072–1081.
- Navarro-Martín, L.; Viñas, J.; Ribas, L.; Díaz, N.; Gutiérrez, A.; Di Croce, L.; Piferrer, F. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011, 7, e1002447.
- Campos, C.; Valente, L.; Conceição, L.; Engrola, S.; Fernandes, J. Temperature affects methylation of the myogenin putative promoter, its expression and muscle cellularity in Senegalese sole larvae. Epigenetics 2013, 8, 389–397.
- Akhtar, W.; Veenstra, G.J.C. TBP-related factors: A paradigm of diversity in transcription initiation. Cell Biosci. 2011, 1, 23.
- Le Luyer, J.; Laporte, M.; Beacham, T.D.; Kaukinen, K.H.; Withler, R.E.; Leong, J.S.; Rondeau, E.B.; Koop, B.F.; Bernatchez, L. Parallel epigenetic modifications induced by hatchery rearing in a Pacific salmon. Proc. Natl. Acad. Sci. USA 2017, 114, 12964–12969.
- Rodriguez Barreto, D.; Garcia de Leaniz, C.; Verspoor, E.; Sobolewska, H.; Coulson, M.; Consuegra, S. DNA methylation changes in the sperm of captive-reared fish: A route to epigenetic introgression in wild populations. Mol. Biol. Evol. 2019, 36, 2205–2211.
- Morán, P.; Marco-Rius, F.; Megías, M.; Covelo-Soto, L.; Pérez-Figueroa, A. Environmental induced methylation changes associated with seawater adaptation in brown trout. Aquaculture 2013, 392, 77–83.
- Wang, S.Y.; Lau, K.; Lai, K.-P.; Zhang, J.-W.; Tse, A.C.-K.; Li, J.-W.; Tong, Y.; Chan, T.-F.; Wong, C.K.-C.; Chiu, J.M.-Y.; et al. Hypoxia causes transgenerational impairments in reproduction of fish. Nat. Commun. 2016, 7, 12114.
- Cheng, Y.; Vechtova, P.; Fussy, Z.; Sterba, J.; Linhartová, Z.; Rodina, M.; Tučková, V.; Gela, D.; Samarin, A.M.; Lebeda, I. Changes in phenotypes and DNA methylation of in vitro aging sperm in common carp Cyprinus carpio. Int. J. Mol. Sci. 2021, 22, 5925.
- Wang, F.-L.; Yan, L.-X.; Shi, H.-J.; Liu, X.-Y.; Zheng, Q.-Y.; Sun, L.-N.; Wang, D.-S. Genome-wide identification, evolution of DNA methyltransferases and their expression during gonadal development in Nile tilapia. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 226, 73–84.
- Wang, Y.Y.; Sun, L.X.; Zhu, J.J.; Zhao, Y.; Wang, H.; Liu, H.J.; Ji, X.S. Epigenetic control of cyp19a1a expression is critical for high temperature induced Nile tilapia masculinization. J. Therm. Biol. 2017, 69, 76–84.
- Ribas, L.; Vanezis, K.; Imués, M.A.; Piferrer, F. Treatment with a DNA methyltransferase inhibitor feminizes zebrafish and induces long-term expression changes in the gonads. Epigenet. Chromatin 2017, 10, 59.
- Skaftnesmo, K.O.; Edvardsen, R.B.; Furmanek, T.; Crespo, D.; Andersson, E.; Kleppe, L.; Taranger, G.L.; Bogerd, J.; Schulz, R.W.; Wargelius, A. Integrative testis transcriptome analysis reveals differentially expressed miRNAs and their mRNA targets during early puberty in Atlantic salmon. BMC Genom. 2017, 18, 801.
- Domingues, W.B.; Silveira, T.L.; Nunes, L.S.; Blodorn, E.B.; Schneider, A.; Corcine, C.D.; Varela Junior, A.S.; Acosta, I.B.; Kütter, M.T.; Greif, G.; et al. GH Overexpression alters spermatic cells microRNAome profile in transgenic zebrafish. Front. Genet. 2021, 12, 1712.
- Gay, S.; Bugeon, J.; Bouchareb, A.; Henry, L.; Delahaye, C.; Legeai, F.; Montfort, J.; Le Cam, A.; Siegel, A.; Bobe, J.; et al. MiR-202 controls female fecundity by regulating medaka oogenesis. PLoS Genet. 2018, 14, e1007593.
- Aydin, F.; Şehriban, Ç.-Y. Effect of probiotics on reproductive performance of fish. Nat. Eng. Sci. 2019, 4, 153–162.
- Salam, M.A.; Islam, M.; Paul, S.I.; Rahman, M.; Rahman, M.L.; Islam, F.; Rahman, A.; Shaha, D.C.; Alam, M.S.; Islam, T. Gut probiotic bacteria of Barbonymus gonionotus improve growth, hematological parameters and reproductive performances of the host. Sci. Rep. 2021, 11, 10692.
- Dimitroglou, A.; Merrifield, D.L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D.; Davies, S.J. Microbial manipulations to improve fish health and production—A Mediterranean perspective. Fish Shellfish Immunol. 2011, 30, 1–16.
- Jayasankar, V.; Tomy, S.; Wilder, M.N. Insights on molecular mechanisms of ovarian development in decapod crustacea: Focus on vitellogenesis-stimulating factors and pathways. Front. Endocrinol. 2020, 11, 1790–1796.
- Gioacchini, G.; Giorgini, E.; Merrifield, D.L.; Hardiman, G.; Borini, A.; Vaccari, L.; Carnevali, O. Probiotics can induce follicle maturational competence: The Danio rerio case. Biol. Reprod. 2012, 86, 65.
- Gao, Y.; Liu, J.; Wang, X.; Liu, D. Genetic manipulation in zebrafish. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2017, 33, 1674–1692.
- Koulish, S.; Kramer, C.R.; Grier, H.J. Organization of the male gonad in a protogynous fish, Thalassoma bifasciatum (Teleostei: Labridae). J. Morphol. 2002, 254, 292–311.
- Sumon, T.A.; Hussain, H.M.A.; Sumon, M.A.A.; Jang, W.J.; Guardiola, F.A.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, E.W.; Kim, C.H.; Hasan, M.T. Functionality and prophylactic role of probiotics in shellfish aquaculture. Aquac. Rep. 2022, 25, 101220.
- Sumon, M.A.A.; Sumon, S.T.A.; Hussain, M.A.; Lee, S.J.; Jang, W.J.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, E.W.; Hasan, M.T. Single and multi-strain probiotics supplementation in commercially prominent finfish aquaculture: Review of the current knowledge. J. Microbiol. Biotechnol. 2022, 32, 681–698.
- Volkoff, H.; London, S. Nutrition and reproduction in fish. Encycl. Reprod. 2018, 9, 743–748.
- Giri, S.S.; Yun, S.; Jun, J.W.; Kim, H.J.; Kim, S.G.; Kang, J.W.; Kim, S.W.; Han, S.J.; Sukumaran, V.; Park, S.C. Therapeutic effect of intestinal autochthonous Lactobacillus reuteri P16 against waterborne lead toxicity in Cyprinus carpio. Front. Immunol. 2018, 9, 1824.
- Gioacchini, F.; Lombardo, F.; Merrifield, D.; Silvi, S.; Cresci, A.; Avella, M.; Carnevali, O. Effects of probiotic on zebrafish reproduction. J. Aquac. Res. Dev. 2011, S1, 1–6.
- Grunwald, D.J.; Eisen, J.S. Headwaters of the zebrafish—Emergence of a new model vertebrate. Nat. Rev. Genet. 2002, 3, 717–724.
- Ye, M.; Chen, Y. Zebrafish as an emerging model to study gonad development. Comput. Struct. Biotechnol. J. 2020, 18, 2373–2380.
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354.
- Xia, Y.; Yu, E.; Lu, M.; Xie, J. Effects of probiotic supplementation on gut microbiota as well as metabolite profiles within Nile tilapia, Oreochromis niloticus. Aquaculture 2020, 527, 735428.
- Ohga, H.; Selvaraj, S.; Matsuyama, M. The roles of kisspeptin system in the reproductive physiology of fish with special reference to chub mackerel studies as main axis. Front. Endocrinol. 2018, 9, 147.
- Yu, G.; Zhang, D.; Liu, W.; Wang, J.; Liu, X.; Zhou, C.; Gui, J.; Xiao, W. Zebrafish androgen receptor is required for spermatogenesis and maintenance of ovarian function. Oncotarget 2018, 9, 24320–24334.
- Valcarce, D.G.; Riesco, M.F.; Martínez-Vázquez, J.M.; Robles, V. Diet supplemented with antioxidant and anti-inflammatory probiotics improves sperm quality after only one spermatogenic cycle in zebrafish model. Nutrients 2019, 11, 843.
- Carnevali, O.; Avella, M.; Gioacchini, G. Effects of probiotic administration on zebrafish development and reproduction. Gen. Comp. Endocrinol. 2013, 188, 297–302.
- Qin, C.; Xu, L.; Yang, Y.; He, S.; Dai, Y.; Zhao, H.; Zhou, Z. Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC 6141 and Lactobacillus casei BL23. Reproduction 2014, 147, 53–64.
- Gioacchini, F.; Maradonna, F.; Lombardo, F.; Bizzaro, D.; Olivotto, I.; Carnevali, O. Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction 2010, 140, 953–959.
- Major, K.M.; DeCourten, B.M.; Li, J.; Britton, M.; Settles, M.L.; Mehinto, A.C.; Connon, R.E.; Brander, S.M. Early life exposure to environmentally relevant levels of endocrine disruptors drive multigenerational and transgenerational epigenetic changes in a fish model. Front. Mar. Sci. 2020, 7, 471.
