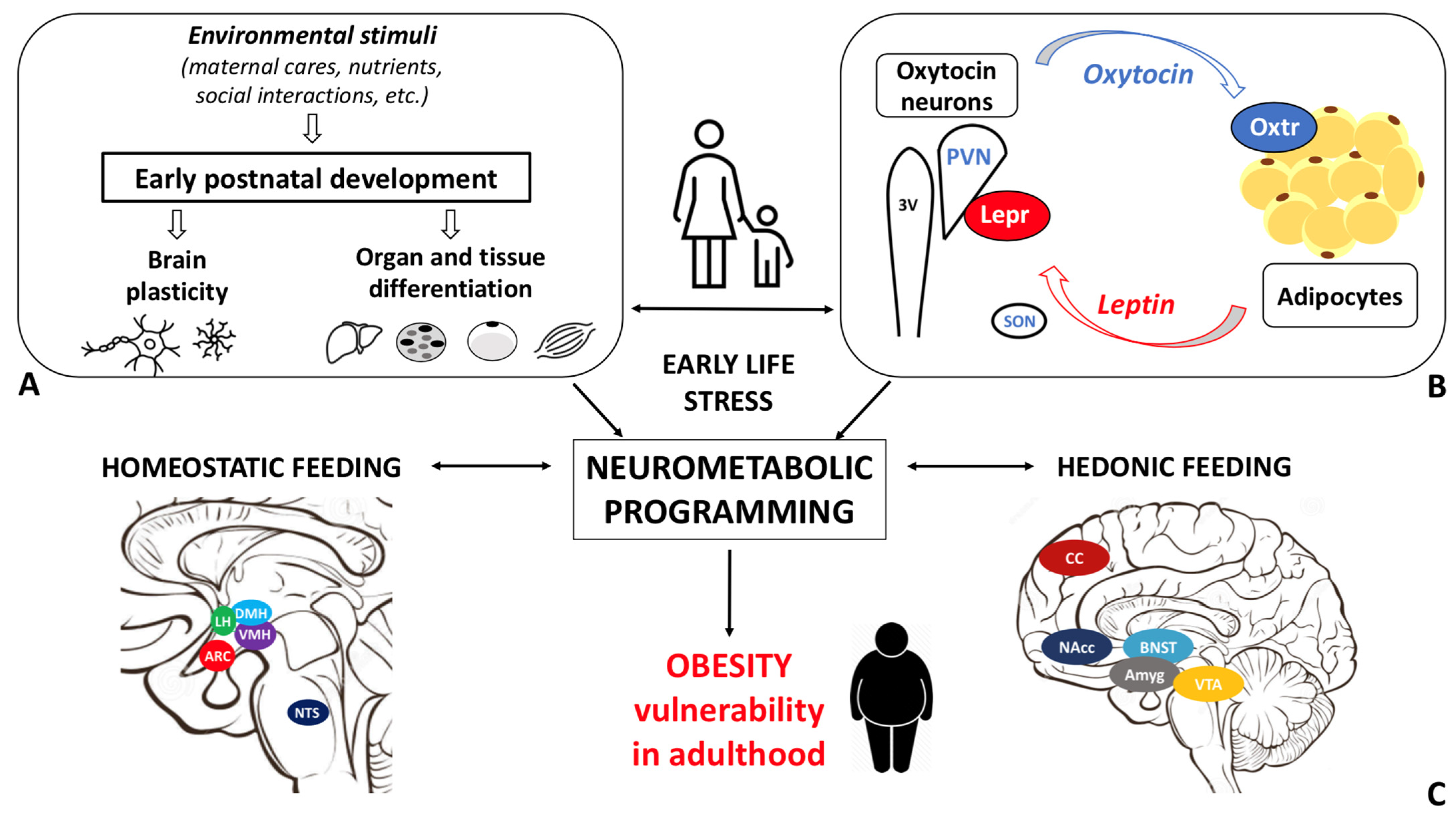
Obesity disease results from a dysfunctional modulation of the energy balance whose master regulator is the central nervous system. Consistently, the prevalence of obesity is higher among individuals who experienced early life stress (ELS). Oxytocin, a hypothalamic neurohormone, regulates the energy balance and modulates social, emotional, and eating behaviors, exerting both central and peripheral actions. Oxytocin closely cooperates with leptin in regulating energy homeostasis. Based on the available data, alterations in the oxytocin system may in part mediate the ELS-induced susceptibility to obesity.
- obesity
- early life stress (ELS)
- oxytocin
1. Obesity: epidemiology, etiopathophysiology and early development
1. Obesity: Epidemiology, Etiopathophysiology, and Early Development
Obesity, defined as a body mass index (BMI) ≥30.0 kg/m2, is a multifactorial, chronic, and relapsing disease that has spread to pandemic proportions during the last decades [1][2][1,2]. Obesity prevalence has in fact nearly tripled since 1975 and its incidence is expected to increase further in the near future [1][2][3][1,2,3]. This estimate is not surprising as the incidence of obesity among children is rising steeply [4] and the condition is usually maintained throughout life [5]. Notably, in 2016, more than 1.9 billion people (39% of adults) worldwide were overweight (25.0 ≤ BMI < 30.0 kg/m2) and more than 650 million suffered from obesity (13%). Furthermore, in 2020, 39 million children under the age of 5 were overweight or suffered from the disease [6]. Obesity is associated with a higher risk of developing over 200 medical complications, including insulin resistance, type 2 diabetes mellitus, hypertension, metabolic syndrome, cardiovascular disease, and several types of cancer. For the above reasons, obesity is recognized as the fifth leading cause of death worldwide and as a major burden for the global healthcare systems [6][7][8][6,7,8]. From a biological standpoint, obesity results from the inability to ensure energy homeostasis, an impairment referred to as energy balance dysfunction. This concept is often simplistically ascribed to excessive energy intake (eating) and low energy expenditure (physical activity), hence to an “unhealthy lifestyle”. Nonetheless, the etiology of obesity is complex and multifactorial [9]. According to the Foresight Study, multiple environmental (i.e., food industry, pollution, education, culture, access to healthcare), psychological (individual and social), and biological (genetic, epigenetic, endocrinological) factors not only contribute to determine the obesity risk, but also positively and negatively influence each other in triggering the disease and its morbidity [9]. Eventually, the interplay between these causal factors results in a dysfunctional regulation of the energy balance, hence abnormal energy intake and expenditure.The modulation of the energy balance occurs in the central nervous system (CNS) [10][11][12][13]. The master regulator is the hypothalamus where all signals from other brain areas and periphery are integrated and translated into specific behavioral, autonomic, and endocrine outputs [10][11][12][14][15]. The crucial role of the CNS in obesity susceptibility is well documented by recent genome wide association studies that implicate pathways related to synaptic function, extracellular matrix composition, and glutamate signaling [16], as well as brain G protein-coupled receptors, as primary in determining BMI variations [17].
The maturation of the central neural circuitries involved in energy balance control is not completed at birth but occurs during early life. In mammals, postnatal ages are denoted by critical developmental periods during which organs and neural systems are highly plastic. In this timeframe, adverse nutritional, social, and environmental cues may program body metabolism to maximize energy accrual to face hostile conditions. Consistently, a higher prevalence of obesity among individuals exposed to early life stress (ELS) during both the pre- and postnatal periods is documented [18].
2. Early life stress
2. Early Life Stress
3. Oxytocin: the neuroendocrine hub of social bonding, stress, eating behavior, and metabolic health Oxytocin: The Neuroendocrine Hub of Social Bonding, Stress, Eating Behavior, and Metabolic Health
In the study of early adversity determined by abnormal infant care, particularly pertinent is the research on the neurohormone Oxt. Produced by neurons located in the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei (Figure 1 Figure 1A-E), Oxt plays a pivotal role in the regulation of a variety of behaviors including social, emotional, sexual, eating, and addiction behaviors [14][21]. Oxt is produced by both magnocellular and parvocellular neurons: the formers are contained in PVN and SON and mainly project to the neurohypophysis where Oxt is released into the circulation, while the latters, mainly contained in the PVN but also scattered in other hypothalamic and extrahypothalamic areas, project to different hindbrain regions e.g., solitary tract nucleus [32][33][59,60]. Interestingly, specialized PVN and SON magnocellular Oxt neurons develop axon collaterals projecting to forebrain limbic regions (e.g., prefrontal cortex, nucleus accumbens, anterior and central amygdala, bed nucleus of the stria terminalis -BNST-(BNST), hippocampus) (Figure 2 Figure 2A-–F), a. This finding revealedhas only ibeen described in advanced vertebrates and thoughtis believed to have developed together with the social and emotional behavioral complexity of species.
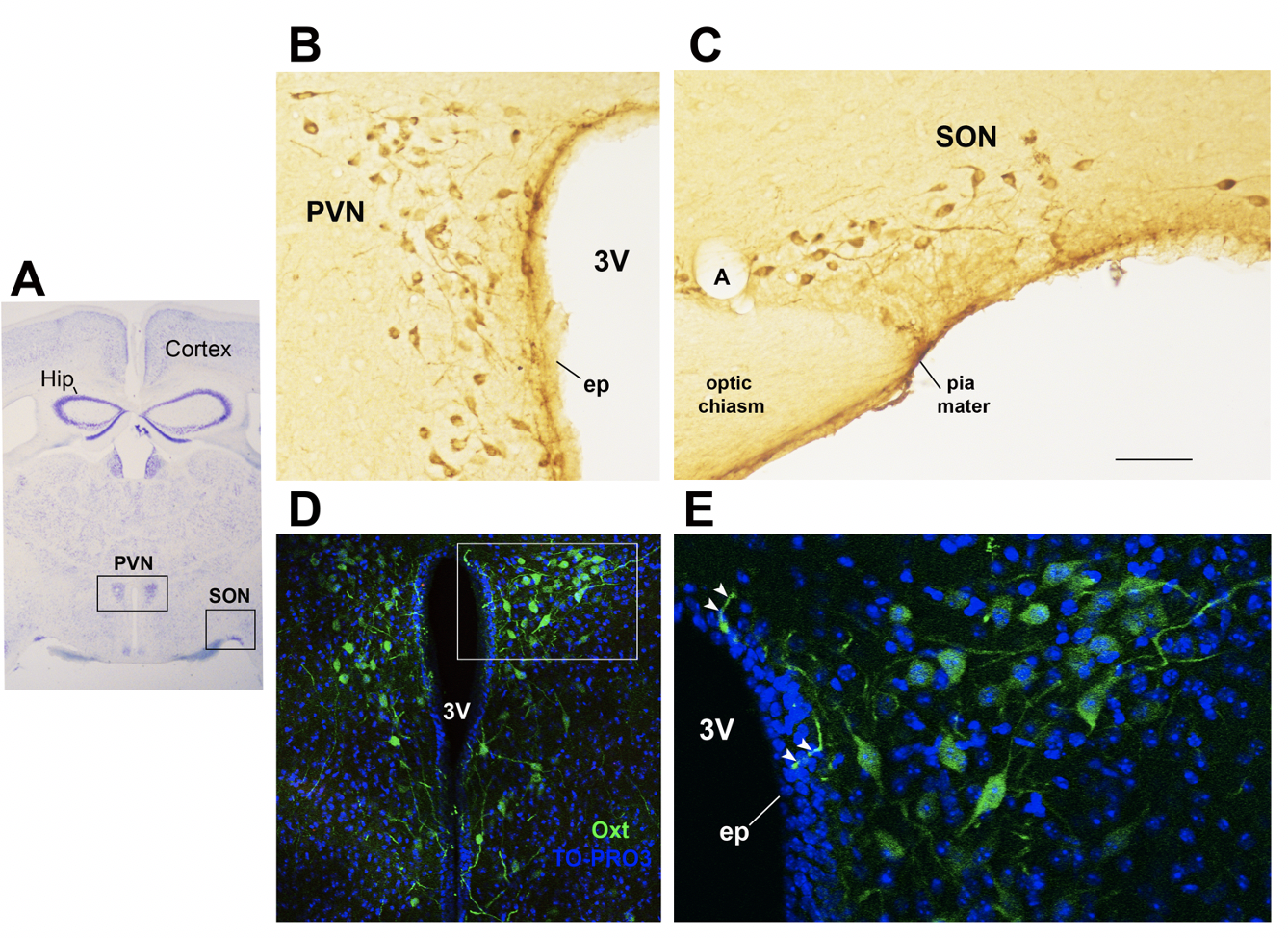
Figure 1. Oxytocinergic neurons in the paraventricular (PVN) and supraoptic (SON) hypothalamic nuclei. (A) Light microscopy (LM): Nissl-stained brain coronal section of the region corresponding to bregma −0.94 mm; PVN: hypothalamic paraventricular nucleus; SON: hypothalamic supraoptic nucleus; Hip: hippocampus. (B) LM: peroxidase immunohistochemistry of oxytocin (Oxt)-positive neurons in proximity of the ependymal layer (ep) of the third ventricle (3 V) in the PVN. (C) Peroxidase immunohistochemistry of Oxt neurons in the SON; A: artery. (D) Double-label confocal microscopy of Oxt neurons (green) and cell nuclei (blue TO-PRO3 staining) in the PVN. Panel (E) is an enlargement of the area framed in (D), showing Oxt-positive neurons and their projections (arrowheads) reaching and contacting the ependymal cells and the cerebrospinal fluid of the third ventricle (3 V). All figures refer to a 6-month-old male C57BL/6 mouse. Bregma reference sections from “The Mouse Brain Atlas”, Paxinos and Franklin (2001). The scale bar is included in C only and corresponds to different μm in each figure as follows: in (A): 1500 μm; in (B,C): 45 μm; in (D): 120 μm; in (E): 40 μm. All figures are original.
Oxt stimulates maternal care, maternal-–infant attachment, and social bonding and is capable of can attenuatinge the response to stress respon, anxiety, and depression [21,28]. It also has a crucial role, displaying anxiolytic and antidepressant properties in the regulation of the stress system by reducing HPA activity and by supporting the parasympathetic nervous system [14][21][28,72,73]. Recent works on the neurobiological basis of attachment, coupled with studies on children adopted from orphanages, suggest that there may be a sensitive period for the development of oOxytocin-t–dopamine connections (particularly in the nucleus accumbes of the striatum), which exerts enduring effects on the neurobiology of social relationships [34][72], for includstance, by strengthening their ability to physiologically buffer stress [35][73].
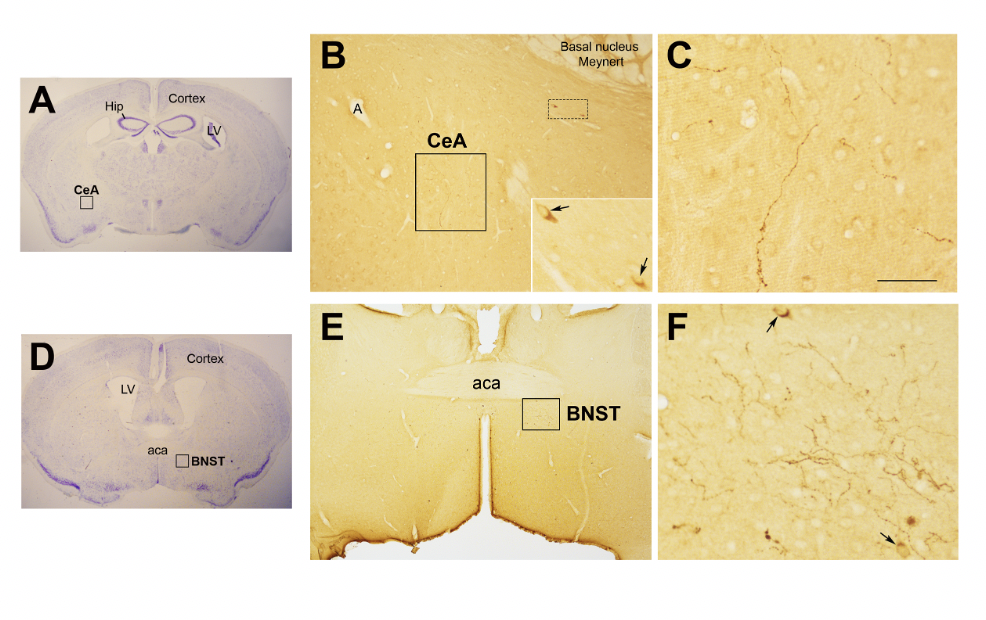

Figure 2. Oxytocinergic projections in the central nuclei of the amygdala (CeA) and in the bed nucleus of the stria terminalis (BNST). (A) Light microscopy (LM): Nissl-stained brain coronal section of bregma −0.94 mm; Hip: hippocampus; LV: lateral ventricle; CeA: central nuclei of the amygdala. (B) LM: peroxidase immunohistochemistry of oxytocin (Oxt), showing oxytocinergic fibers (framed area) and two parvocellular Oxt neurons (dotted framed area) in the CeA. Inset: enlargement of the dotted framed area, where Oxt-positive neurons are indicated by arrows. (C) Enlargement of the area framed in (B), rich in Oxt-positive fibers. (D) LM: Nissl-stained brain coronal section of the bregma 0.02 mm; BNST: bed nucleus of the stria terminalis; aca, anterior commissure. (E) LM: peroxidase immunohistochemistry of oxytocinergic projections in the BNST. (F) Enlargement of the area framed in (E) showing Oxt neurons (arrows) and Oxt projections in the BNST. All figures relate to a 6-month-old male C57BL/6 mouse. Bregma reference sections from “The Mouse Brain Atlas”, Paxinos and Franklin (2001). The scale bar is only specified in C and corresponds to different μm in each figure, as follows: in (A,D): 2300 μm; (B): 200 μm, inset 50 μm; in (C): 50 μm; in (E): 300 μm; in (F): 50 μm. All figures are original.
Interestingly, Oxt also attenuates addictive behaviors and inhibits appetite [34][72]. Consistent with these data, there is a mounting body of evidence pointing at Oxt role in promoting weight loss and ameliorating obesity-related metabolic dysfunctions [22][36][37][38][29,64,74,75]. Intranasal Oxt administration is currently being tested for the treatment of obesity as this route facilitates an increase in the central concentrations of the nonapeptide through channels surrounding the trigeminal and olfactory nerve fibers [39][40][41][76,77,78]. Considering the complex and multiple functions of Oxt, which affects aspects as diverse as mother–infant bonding, eating behavior, and stress response, its potential role in determining the impact of ELS on eating behavior and metabolic health deserves further investigation.
4. Oxytocin system: early development and impact of ELS
5. Oxytocin System: Early Development and Impact of Early Life Stress
Studies conducted on rodents demonstrated that earAly adverse experiences could have a profound impact on the way the oxytocin system is shaped. Oxt neurons progressively increase from PND 2 to 21, reaching maturation by the second postnatal week [42]. tered On the other side, oxytocin neuron axons reach their targets only at early adulthood, when the Oxt receptor (Oxtr) is already widely expressed in different regions (detectable from embryonic day 14 in females and at PND 2 in males) [33]. Importatr expression and bindintly, Oxt synthesizing neurons play a fundamental role in their own maturation: local Oxt release activates Oxtr expressed on Oxt neurons resulting in further Oxt discharge, a phenomenon that evidences how variation in Oxt levels during this period can deeply impact these neurons maturation [14][43][42]. It is possib in response to changes in maternale that postnatal environmental stimuli affects Oxt production, hence the development of the Oxt system, with the final objective to retain only functional pathways necessary to face external conditions. This phenomenon may also explain the interindividual variability in Oxt neurons projection revealed by studies mapping the Oxt system [44]. In addition, different spatio-temporal Oxtr expression patterns during development have care were describeen described in males and females, suggesting a distinct and sex-specific sensitivity to Oxt levels during critical periods [33].
Alteration in Oxtr expression and in several binding have been detected in several brain regions in response to variation in maternal care in different animal models [29][45][36,79]. Maternal high licking and grooming (LG) result in increased Oxt expression at PND13, while low LG leads to reduced Oxtr protein levels and receptor binding in several female’ss’ brain regions (e.g., PVN, central nucleus of the amygdala) [29][46][45][36,69,79]. AlFurthough, Tsuda et al., documenermore, while one study detected a higher number of Oxt -positive cells in the PVN of adult male mice exposed to MS [47][80], other investudies rigations with a similar protocol reported a lower number of these cells in the SON and PVN f[71,73,81]. In additiolln, higher Oxtr expressiowing a similar protocoln and a higher number of Oxt projections to [48][35][49].the Oxbasolat changes in response to eeral amygdala were described in male mice exposed to MS [81]. Early adversity are generusually in the direction of bluntresults in reduced circulating Oxt levels [35][73]. However, Oxt levels may actually ricse in response to a prolonged exposure to adverse stimuli , possibly to protect the system from the deleteriousharmful effects of stress [35][73].
A meta-analyses performed by Ellis and colleagues documented lower plasma OXT levels and reduced or negative response to OXT intranasal administration among individuals who experienced childhood adversity [50][83]. In addition, an unsecure attachment style was associated with lower OXTR expression in the peripheral blood mononuclear cells of women [51]. Based on this evidence, early experience shapes the adult Oxt system and manipulation of maternal care from infancy confers enduring changes in the Oxtr expression profile, a phenomenon whose mechanisms and implications are not fully elucidated. In addition, early stress in childhood may be produced by overexposure to blue light of digital device screens affecting sleep patterns in children as young as 2 years old, as discussed recently in [56].
Recent studies documented the association between the alteration in the Oxt system induced by ELS and the development of cardiovascular diseases [52][53][54]. However, little is known about the link between Oxt, ELS, and metabolic health. While data on the relationship between ELS and Oxt and between ELS and metabolic health are scanty, evidence linking ELS to metabolic health, focusing on the Oxt system, is not documented in the literature. Specifically, it is unclear how ELS induces changes in the action of and sensitivity to Oxt and how these changes influence adipose tissue development, energy balance, metabolic health, and feeding behaviors in adulthood. The sex-dependent differences in the Oxt system may affect the adipose proliferative niches (hence, adipose tissue development) and may partly contribute to the distinctive fat mass distribution and susceptibility to obesity comorbidities described in males and females. Furthermore, while the effect of Oxt manipulation in early development has been widely investigated [42], little is known about the function of endogenous Oxt at such a time [23]. Several lines of research have demonstrated how spatio-temporal Oxtr expression varies in response to environmental cues, especially during critical periods. It is widely accepted that Oxtr may be a developmental plasticity gene that acts as a transducer of the social environment to finetune the experience-dependent plasticity of the social brain [33]. Therefore, Oxtr expression and distribution, as opposed to circulating Oxt levels per se, may be of particular significance. As noted above, Oxt influences reward-related behaviors in different ways: while it reduces motivated behavior toward palatable food, it modulates social rewards and attention-orienting responses to external social cues [55]. It is reasonable to hypothesize that dysfunctional social attachment to the parental figure during early development (MS or LN) alters the oxytocinergic system maturation such that the reward response to feeding prevails on the reward response to social cues. This paradigmatic model may be employed not only to study obesity vulnerability, but also psychiatric diseases, such as eating disorders, depression, and addiction. Indeed, the limitations related to the use of animal models for the study of such complex diseases should be acknowledged. Growth in an unpredictable environment, where maternal care (feeding) is not constantly ensured, may result in a neurometabolic programming that maximizes energy accrual and minimizes its waste. As a result, besides the enhanced reward value of food, the ability to store energy to cope with an adverse environment may also be increased. Deeper investigation of the biological underpinnings of ELS-induced metabolic and eating behavior abnormalities is warranted to characterize unexplored mechanisms responsible for obesity and to identify novel preventive and/or therapeutic strategies.
5. Early LSife Stress, Oxytocin, Eating Behavior, and Metabolic Health: Future Research Directions
| 1 | Do the Oxt and Leptin systems influence each other’s development? |
| 2 | Is the Oxt–Lep systems interaction impacted by obesity? |
| 3 | Is Oxt the mediator of ELS-induced changes in metabolic health? |
| 4 | What are the consequences of ELS on short- and long-term eating behaviors, such as chow vs. palatable food consumption? |
| 5 | What are the consequences of ELS on total weight changes and metabolic health, such as adipose tissue development and resting energy expenditure? |
| 6 | What are the consequences of ELS on the vulnerability to an obesogenic environment in adulthood, such as adipose tissue dysfunction and metabolic abnormalities? |