Fish represent an excellent source of animal protein as well as a biomedical research model as a result of their evolutionary relatedness and similarity with the human genome. Commercial and ornamental fish culture has achieved popularity, but reproductive dysfunctions act as a limiting factor for quality fry production, interfering with the sustainability of the aquaculture industry. Fish reproduction is crucial for any species’ existence, and reproductive performance can potentially be improved through applications of epigenetics and probiotics. Epigenetics is a highly sensitive molecular approach that includes chromatin structure and function alteration, DNA methylation, and modification of non-coding RNA molecules for the transfer of desired information from parents to offspring. DNA methyltransferase improves reproductive cyp11a1, esr2b, and figla gene expression and feminizes zebrafish (Danio rerio). Moreover, epigenetics also contributes to genome stability, environmental plasticity, and embryonic development. However, methylation of specific genes can negatively affect sperm quality, resulting in poor fertilization. Probiotic administration is able to induce responsiveness of incompetent follicles to maturation-inducing hormones and can change oocyte chemical composition during vitellogenic development. The positive role of probiotics on testicular cells is validated by upregulating the transcription levels of leptin, bdnf, and dmrt1 genes facilitating the spermatogenesis.
1. Introduction
Aquaculture has been known for millennia, when the trend of captive fish rearing began, and it is now playing a crucial role in solving the world food crisis, particularly in meeting the protein demand
[1]. However, broodstock management, which includes the optimization of critical reproductive processes, such as nutrition, maturation, egg and sperm production, and spawning, remains a major obstacle to the advancement of the aquaculture industry. Captive broodstocks are most vulnerable to the disruption of reproductive activities by hormonal imbalance and unfavorable environmental parameters
[2] resulting in reproductive dysfunction. Such reproductive impairments include no or poor-quality egg or sperm production, defective or weak spawn, less growth and high mortality of fry, and sometimes mortality of the brood. Reproductive dysfunctions can be triggered by direct or indirect variations of the gametes and endocrine system, captivity-induced stress, unsuitability of the spawning environment, and deficiencies in the nutritional profiles of feeds
[3][4][3,4]. Some taxonomic groups of fishes are far more sensitive to such inhibitory influences than others. Reproductive dysfunction is common in
Anguilla spp. (catadromous eels),
Seriola spp. (greater amberjack and Japanese yellowtail),
Caranx ignobilis (giant trevally),
Epinephelus spp. (groupers) and
Thunnus spp. (bluefin tuna)
[5],
Anoplopoma fimbria (Sablefish)
[6], and
Oncorhynchus mykiss (Rainbow trout)
[2].
To avoid reproductive dysfunction, scientific management of broodstock animals ensures proper physiology, immunology, reproductive enzymes pathways activation, and transcription of specific reproductive genes. Many commercial farms seek to maintain healthy broodstocks by supplementation with probiotics to increase reproductive enzymes activities and gene transcription, but dysfunction remains fairly common during breeding seasons
[7][8][7,8]. Moreover, epigenetic mechanisms include DNA methylation, histone modification, and noncoding RNA action underlying various processes have recently received considerable attention in aquaculture and offer some promise in the amelioration of breeding performance
[9].
The term epigenetics literally means “above” or “on top of” genetics, and epigenetic traits refer to stable heritable phenotypes resulting from changes in the status of chromosomes without alteration of DNA sequences
[10]. Epigenetic reprogramming underpins many developmental processes such as gametogenesis and embryogenesis, foundation of environment regulating events of fish sex differentiation, providing a linkage between phenotypic and metabolic changes during domestication
[11]. In several studies, epigenetic inheritance was found to be more feasible in fish as compared with terrestrial animals
[12][13][14][12,13,14]. Epigenetic information has the potential to contribute to lower disease prevalence and the potential eradication of the use of antibiotics in commercial aquaculture
[15]. Sperm quality has been linked to DNA methylation in spermatozoa and in
Morone saxatilis (striped bass) sperm DNA methylation has a positive relationship with male reproductive capacity
[16]. Epigenetics influence fish sex determination and differentiation, facilitating interactions between these processes with other surroundings
[17]. When it comes to valuable fish species, such as grouper, it is essential to know sex patterns, especially when their sex is changed. In aquaculture, a stable and predictable mating system is critical for healthy fry production
[9] and alterations in DNA methylation patterns triggered by higher temperatures can lead to more masculine traits in females
[18][19][18,19]. Although epigenetics has huge potential, studies on economically important aquaculture species remain preliminary, and many unanswered questions remain
[12][20][12,20]. An understanding of epigenetic mechanisms for commercial species could contribute to expansions and improvements in the economic viability of large-scale aquaculture.
Aquaculture technology has developed a positive view of probiotics application as an alternative to the application of synthetic antibiotics or chemicals
[21][22][23][21,22,23]. Recognition of advantages of practical use of probiotics has grown in light of evidence of upregulation of fish growth, stress adaptation, immune modulation, and disease resistance
[24][25][24,25]. Probiotics are defined as “live microorganisms administrated at an appropriate concentration that exert beneficial effects on host health and immune parameters”
[26]. Ghosh, et al.
[27] initially reported probiotics capacity to restore viable nutrients in female live-bearing ornamental fishes such as
Poecilia reticulata (Guppy),
P. sphenops (molly),
Xiphophorus helleri (Swordtail) and
X. maculatus (Platyfish). A later investigation was also carried out in
X. helleri [28],
Danio rerio (Zebrafish)
[29][30][31][32][33][34][29,30,31,32,33,34],
Carassius auratus (Goldfish)
[35], and
Fundulus heteroclitus (Killifish)
[36]. Limited probiotics studies were also conducted to improve reproductive performance in commercial fishes such as
Oreochromis niloticus (Nile Tilapia)
[37],
O. mykiss [38],
Ompok pabda (Butter catfish)
[39],
Clarias gariepinus (African catfish)
[40] and
A. anguilla (European eel)
[41] on the subject of reproductive indicators such as fecundity (Fec), hatching rate (HR), gamete quality, gonadosomatic index (GSI), fertilization rate (FR), and survival rate (SR). Pathogenic infections often leading to mortality of brood or offspring may be unnoticed by hatchery managers. Synthetic antibiotics or chemical applications to control infection may lead to mass environmental bacterial killing, antibiotic deposition in fish body and the generation of antibiotic-resistant pathogens
[42][43][42,43]. As a result, probiotics may be a favorable option for broodstocks and fry for the control of infections and for improved reproductive success
[44]. The use of probiotics helps to stabilize and diversify the intestinal microbial community, leading to improved reproduction processes through activation of different hormones, enzymes, and genes transcription resulting better FR, HR, SR, and larval growth
[7][38][7,38].
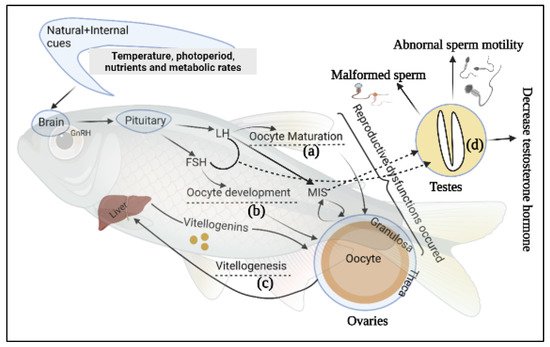
2. Fish Reproductive Dysfunctions
Globally, aquaculture is continually striving for the consistency of physiological integrity of fingerlings through standardized reproductive programmes, which are a key objective for sustainable aquaculture
[45]. Brood fish rearing and their management strategies have been considered as vital for aquaculture production for the last three decades. Generally, many species of captive fishes do not perform normal reproduction (especially females), a phenomenon potentially caused by a lack of natural spawning stimuli, specifically failure of oocyte maturation
[46][47][46,47]. Supplied protein, fatty acids, lipid, vitamins, especially E and C, and carotenoids also influence fish Fec, FR, HR, and larval development
[48][49][48,49]. Moreover, presence of chemical fertilizers, antibiotics, hormones and industrial effluents, environmental degradation, and frequent fluctuation of temperature in nature and captivity suppress the immunity and beneficial microbial activity in broodstock fishes, making them more susceptible to infectious disease
[27][50][51][52][27,50,51,52]. Hypothalamus, pituitary, and gonads form the hypothalamic-pituitary-gonadal (HPG) axis, which regulates reproductive function in most fishes
[53]. The hypothalamus and the pituitary glands modulate the production of pituitary gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH), and gonadal sex steroids (SS) regulate vitellogenesis and oocyte maturation. FSH and LH have been confirmed to figure importantly in oocyte growth and maturation
[54]. Gioacchini et al.
[7] have categorized three types of female broodstocks reproductive dysfunctions based on the affected pattern of reproductive cycle whereas, Selvaraj, et al.
[55] documented two modes of dysfunction of teleost fishes (
Figure 1). Both groups of investigators associated the most disruptive reproductive dysfunctions occurred with the vitellogenic phase, as exemplified by failed vitellogenesis in
Anguilla spp. and
Seriola spp.
[56] and
Mugil cephalus (Grey Mullet)
[57]. The second mode is categorized as the inability to reach final oocyte maturation to ovulation as seen in farmed white and striped bass
[58][59][60][58,59,60] and in Cyprinidae
[61]. The third set of dysfunctions leads to failures to spawn in the breeding season; sometimes
E. aeneus,
M. saxatilis, and
Dentex dentex (common dentex) females may release eggs after ovulations without exhibiting characteristic breeding behavior
[7][62][7,62]. Under some circumstances, cultured salmonids fail to complete the reproductive cycle and eggs in the abdominal cavity are reabsorbed over the following months
[63]. These are examples of failures among female broodstocks that do not appear to be accompanied by reproductive problems that can be attributed to male fishes.
One common problem faced by cultured male fishes is low quantity or quality of milt production during spermiation
[60]. The presence of both male and female germ cells in the testis tissue is referred to as an intersex state caused by endocrine disruption through chemical exposure and hormonal imbalance
[64]. The histopathological assessment of gonadal tissue during the development stage allows the detection of intersex reproductive dysfunction in male Japanese medaka (
Oryzias latipes)
[65].
Figure 1. Schematic representation of the reproductive system and gonadal dysfunctions of fish. The broken line indicates the reproductive developmental stages. These dysfunctions may occur: (a) Lack of effectivity of luteinizing hormone (LH) for oocyte maturation; (b) outreaching of follicle-stimulating hormone (FSH) for oocyte development; (c) lower or no production of vitellogenin from the liver; (d) malformation of sperm and its motility, lowered testosterone to the testis. GnRH: gonadotrophin releasing hormone, MIS: maturation-inducing steroid.
3. Epigenetics Mechanism and Modifications of Fish Reproductive Performance
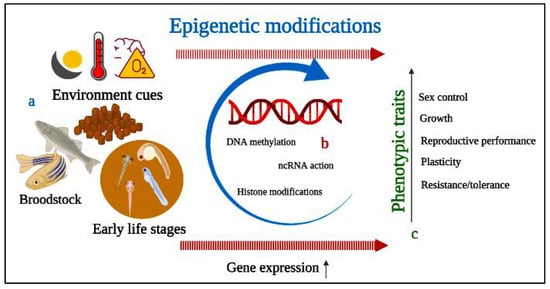
Considerable attention is focused in aquaculture on brood rearing, breeding, feeding, sex control, and disease management. The study of epigenetic mechanisms underlying various molecular mechanisms has the potential to contribute to favorable hatchery productivity. Environmental cues can cause phenotypic changes in organisms via epigenetic mechanisms (
Figure 24). Moreover, environmental temperature, dissolved oxygen, water, pH, pollutants, and other factors influenced epigenetic changes in fish gonad and sex-dependent behavior
[11][66][67][11,131,132]. Epigenetic modifications include DNA methylation, histone modification, chromatin re-modelling, and the action of noncoding RNAs (ncRNAs), attracting interest for their practical potential in fish reproduction
[12].
The structure and consequently the function of chromatin can be altered by epigenetic mechanisms, resulting in the modulation of patterns of gene expression
[68][133]. DNA methylation is the most well-known and well-understood epigenetic mechanism, in response to variable environmental factors, such as photoperiod, toxins, temperature, nutrients, etc.
[69][134]. This entails the addition or removal of a methyl group to DNA, which alters gene function and transcription level as well. The methyl group is covalently added to the 5-carbon location of the cytosine ring, resulting in 5-methylcytosine (5-mC), known colloquially as the “fifth base” of DNA. DNA methylation takes place at CpG doublets either within CpG islands (dinucleotide CG), intergenic regions, or the gene body
[70][135]. It has been reported that CpG islands influence gene expression by regulating transcription factor binding with chromatin structure. De novo DNA methylation and its maintenance are carried out by a family of DNA methyltransferase enzymes (DNMTs) and during embryogenesis, DNMT3A and DNMT3B are responsible for de novo methylation
[71][136]. DNA demethylation is also equally important and accompanied by DNA methylation which is necessary for epigenetic reprogramming of genes. Through the incorporation of histone variants and post-translational modification of histones, chromatin structure can be altered to enhance or repress transcription. These altered chromatin states can be inherited both mitotically and meiotically, suggesting that they may transmit epigenetic information to the next generation. Evidence suggests that certain modified histones are retained non-randomly during spermatogenesis in both mammals and zebrafish, and these marks are thought to play a role in transferring epigenetic information to embryos
[72][73][137,138]. The ncRNAs, which are made up of small and long RNA molecules, can influence gene expression
[74][139]. Investigations reveal that ncRNAs play important roles in genome stability, environmental plasticity, and embryonic development
[75][76][140,141]. The majority of research on ncRNAs in fish and shellfish, including important aquaculture species, has been conducted in Atlantic salmon and rainbow trout
[77][78][142,143]. Aside from developmental programming, broodstock holding/conditioning is an important consideration for the potential transmission of epigenetic information transfer from parents to offspring
[79][144]. Importantly, epigenetic transmission can occur on both the maternal and paternal sides
[80][145] and the study of transgenerational plasticity in fish has grown in popularity
[81][146].
Recent investigations have analyzed the impact of epigenetics, specifically DNA methylation on breeding dynamics and productivity in finfish aquaculture. DNA methylation patterns have been found to change in response to temperature increases, leading to the masculinization of genetically female
D. labrax [82][147],
Cynoglossus semilaevis (Halfsmooth tongue sole)
[18][19][18,19]; a trait which can be passed to offspring. In another study, Campos, et al.
[83][148] found that
Solea senegalensis (Senegalese sole) larvae undergoing metamorphosis had higher methylation levels on the
myog promoter in skeletal muscle when reared at lower temperatures (15 °C). Hatchery offspring grown in captivity acquired epigenetic alterations in sperm which may explain rapid genetic and phenotypic alterations in the hybrid fishes. The differential methylation in hatchery salmon displayed the presence of their TATA-binding protein (a transcription factor that binds specifically to a DNA sequence) during spermiogenesis and embryonic development
[84][85][86][149,150,151]. Epigenetics and broodstock nutrition also influence sperm production and quality in the aquaculture industry. Diet-induced methylation influence freshwater and saltwater trout and higher salt-containing diets caused dramatic changes in global methylation patterns
[87][152]. Moreover, hydrogen sulfide stimulates DNA methylation in environments that can be continued generationally through the germline even after withdrawal of this toxic chemical
[14]. Hypoxia-induced reproductive impairment of gonadal development, low sperm count, and motility through different methylation in sperm genes are inherited by the next generation
[88][153]. Transgenerational epigenetic alteration in spermatozoa of aquatic animals resulted in phenotypic variation and methylation of specific gene groups, with potential negative effects on sperm quality and fertilization in male striped bass
[16].
Global and gene-specific methylation in spermatozoa significantly affect the fertilization performance of
C. carpio, in which methylation at CpG sites markedly increased and decreased after 24 and 96 h of post stripping, respectively
[89][154]. In Nile tilapia, the gonadal transcriptomic study demonstrated that all of the DNMTs were expressed in both male and female reproductive organs while specific DNMTs were more highly expressed in the testis. Incubation of gonads with DNMTs inhibitor showed downregulated DNMTs with increased expression of male and female sex determinant gene
dmrt1 and
cyp19a1a, respectively
[90][155].
It has also been revealed that
cyp19a1a expression in Nile tilapia was critically controlled by environmental factors like temperature
[91][156]. Similarly in zebrafish, DNMTs treatment feminizes the fish, stimulating long-term expression of key reproduction-related genes (e.g.,
cyp11a1,
esr2b and
figla)
[92][157]. However, integrative transcriptome of Atlantic salmon testis revealed the involvement of differentially expressed micro (mi) RNA, thereby resulting in early puberty. This was the first study to link specific groups miRNA involvement in testis maturation in an inversely correlated relationship with targets
[93][158]. Moreover, the endogenous non-coding RNAs (MicroRNAome) of sperm are affected by overexpression of growth hormone and consequently reduced sperm quality and fertilization potentiality of transgenic zebrafish
[94][159]. Finally, the insufficiency of miR-202 compromised oogenesis or folliculogenesis, and significantly decreased the number of follicles
[95][160].
Figure 24. A diagram depicting the potential applications of epigenetics for modulation of reproductive performance. (
a) Environmental cues can alter gene transcription via epigenetic mechanisms; (
b) DNA methylation, histone modifications, and non-coding RNA action resulting in phenotypic changes; (
c) outcomes of epigenetics in phenotypic traits from early to later life stage.
4. Influence of Probiotics on Reproductive Performance of Fish
Host-derived probiotic bacteria are being used increasingly in the aquaculture sector. The host-associated probiotics have not only improved the reproductivity of fish
[96][167] but also helped to improve the endocrine and reproductive signaling of fish. Isolated
Enterococcus xiangfangensis,
Citrobacter freundii,
Pseudomonas aeruginosa,
P. stutzeri, and
B. subtilis from fish resulted in growth, hematological, and reproductive performance upregulation among host fishes
[97][168].
The action mechanism of probiotics on reproductive function is normally indicated by increasing egg production along with improvements in the vitellogenic follicles and GSI
[98][166]. However, the lipidic and glucidic components elevated during pre-vitellogenesis result in oocytes maturation, modification of the secondary structure of protein, and impacts on hydration and phosphorylation
[99][169]. Probiotic administration can induce the responsiveness of incompetent follicles (stage IIIa) to MIH in the maturation period and changes oocyte chemical composition, fostering the vitellogenic development
[100][170]. Modifications of the electrophoretic pattern at maturation, and, to a lesser extent, at yolk protein levels have changed the ooplasma components
[101][171]. In fish, probiotics inhibit apoptosis and increase the rate of follicular survival during the developmental stage. Furthermore, effects of probiotics also increase the production of sperm from the testis, with stimulatory actions on two components known as intertubular (or interstitial) and tubular sections
[34]. Some probiotics have been shown to stimulate steroidogenic Leydig cells, blood/lymphatic vessels, macrophages and mast cells, neural, and connective tissue cells. This is the case although the somatic Sertoli cells and the germ cells are found at different stages of development and Sertoli cells function in the determination of spermatogenic capacity
[102][172]. The close and continuous interaction with Sertoli cells may contribute to germ cell survival. Consequently, the positive role of probiotics in molecular parameters in testicular cells is validated by upregulating the transcription level of leptin, bdnf and dmrt1 genes facilitated the potentialities of spermatogenesis
[41]. Moreover, increased transcription levels of activin, arα, arβ, pr1, and fshr contribute to sperm quality improvement during spermatogenesis
[41].
Probiotics are widely used as feed enrichment for farming aquatic organisms especially fish. The initial application of probiotics in aquaculture was for growth promoters and fish health. However, new areas of research, such as their effect on reproduction, maturation, and fecundity, have been found, though these require more comprehensive development
[96][167]. Several studies have found that probiotics improve host microbial balance and thus improve health, disease resistance, growth performance, feed utilization, and reproductive performance
[96][103][104][167,173,174]. Nutrient supplements for broodstock are critical for aquaculture success. Major nutrients such as lipid, protein, fatty acids, vitamins E and C, and carotenoids are essential for various reproduction processes such as fecundity, fertilization, hatching, and larval development
[49]. In general, studies in fish show that decreased food availability or starvation causes gonad regression and a decrease in female spawning and egg production, whereas increased food availability and supplemented adequate nutrients promote growth and larger body sizes, causing earlier maturation and higher fecundity in some species
[105][116]. However, the administration of different growth promoters (hormones, antibiotics, nutrient mixtures) can cause suppression of the beneficial microbial activity in the intestinal tract of the broodfish. In such conditions, probiotic supplements can be used to repair these deficiencies and improve the fish health conditions in breeding time.
Additionally, supplemented probiotics such as lactic acid bacteria improved feed utilization and reproductive function of fish
[106][175]. The administration of probiotics through feed supplementation could regulate and modify the expression patterns of genes or hormones responsible for the regulation of fish reproduction
[107][176]. Therefore, using probiotics as feed supplements can potentially improve reproductive function and activate reproductive genes to correct reproductive dysfunction.
4.1. Ornamental Fish
Ornamental fish have popularity for their unique colors and their importance is increasing not only for hobby purposes, but also for their use as animal models for scientific research. A diverse array of probiotics induces beneficial effects on ornamental fish reproductive performance, as presented in
Table 12.
4.1.1. Male
Ornamental fish especially, zebrafish were used over the last 20 years for studying genetics and gonadal development
[108][109][177,178]. Probiotics have been used in zebrafish trials to observe the transcription of the genes related to reproductive maturity and reproduction
[110][111][179,180]. Transcription of KiSS1, KiSS2, and gnrh3 genes in the brain can trigger the reproductive system of male fish by the direct or indirect secretion of many hormones in response to probiotics, especially leptin
[112][124]. Valcarce et al.
[34] first reported
P. acidilactici supplementation correlates with and upregulates male reproductive performance of zebrafishes. This probiotic triggered five genes, including brain-derived neurotrophic factor (bdnf), BCL2-interacting killer (bik), double-sex and mab-3 related transcription factor 1 (dmrt1), and FSH beta subunit, and leptin a (lepa) transcription, as an indication of good sperm quality (SQ) marker. These genes all produce positive effects on testicular cells, potentially improving reproductive performance
[34][113][34,181]. The blended dietary administration
P. acidilactici (0.2%) and nucleotide (0.5%) demonstrated positive effects on SQ, sperm motility (SM) and sperm density (SDn) in goldfish (
C. auratus)
[35]. Furthermore,
Lab. rhamnosus and
Bifidobacterium longum have been showing antioxidant and anti-inflammatory features to assist zebrafish SQ and male reproductive behavior
[114][182]. These probiotics demonstrated a positive effect on SQ, SDn, total and progressive SM, and fast spermatozoa subpopulations.
4.1.2. Female
Probiotic
Lab. rhamnosus IMC 501 has striking effects on the ovarian development of female zebrafish
[7][31][7,31]. Moreover, in this fish, dietary administration of
L. rhamnosus at 10
6 CFU g
−1 improved Fec, GSI, and oocyte maturation (FD and FM), supporting reproductive performance by improving fecundity
[29][101][29,171,177. This probiotic increases the transcription level of transforming growth factor b1 (tgfb1), growth differentiation factor9 (gdf9), and bone morphogenetic protein15 (bmp15) contributing to oocyte development of that fish. Carnevali, et al.
[115][183] also monitored the long-term effects of the same probiotic on zebrafish and reported dietary effectiveness on FD; ovulated oocytes quantification; embryo quality and larval growth performance. This probiotic stimulates sexual maturation of this species by improving the expression of aromatase cytochrome p 19 (
cyp19a), vitellogenin (vtg), an isoform of the E2 receptor (era), LH receptor, 20-b hydroxysteroid dehydrogenase (20b-hsd), membrane progesterone receptors a and b, cyclin B, activinbA1, smad2, tgfb1, gdf9 and bmp15, which are responsible for regulating reproductive hormone secretion. Autophagy was observed during follicle development in the ovarian tissue and
L. rhamnosus has a key role in follicle maturation as confirmed by focal plane array analysis
[30]. Miccoli et al.
[33] conducted an experiment with the same probiotic including similar dose, duration, and species as reported by Gioacchini et al.
[30], and reported that probiotics promote embryonic development by changing both maternal and zygotic mRNA levels. Similarly, the use of
L. rhamnosus CICC 6141 and
L. casei BL23 probiotic effects on
D. rerio were documented and later probiotic markedly improved Fec, OVr, HR, and FR
[116][184].
Probiotic,
L. rhamnosus IMC 501 treatment has profound effects on killifish (
Fundulus heteroclitus) GSI, Fec, and embryo SR
[36]. Dietary supplementation of probiotic positively improves egg and ovum diameter, absolute fecundity, and some other properties in goldfish
[35]. Probiotic administration caused a substantial impact on reproductive performance in four live-bearing ornamental species:
Poecilia reticulata,
P. sphenops,
X. helleri, and
X. maculatus [27]. In these fishes, administration of
B. subtilis for 1-year improved GSI, Fec, and fry production of spawning females. Additionally, probiotic could synthesize vitamin B1 and vitamin B12 that controlled the mortality or body deformities of fry. The live-bearing ornamental female swordtails displayed improved GSI, Fec, and fry production after taking commercial probiotic (PrimaLac) (see
Table 12) as a feed additive for 182 days
[28].
Table 12.
Dietary supplemented probiotics effects on ornamental fish reproduction. Symbol: no change (→); increase (↑); decrease (↓) versus controls.
| Supplemented Probiotics |
Fish Species |
Fish Number |
Duration |
Concentration |
Effects on Fish |
References |
| Bacillus subtilis |
Poecilia reticulata (Guppy), P.sphenops (Valenciennes), Xiphophorus helleri (Swordtail fish) and X. maculatus (Platyfish) |
60 virgin females of each species |
365 days |
5 × 107–5 × 108 CFU g−1 and 5 × 105–5 × 106 CFU g−1 |
EP Fec and GSI ↑; SR (fry) ↑; Fry death and deformities ↓ |
[27] |
| Lactobacillus rhamnosus IMC 501 |
Danio rerio (Zebrafish) |
10 females |
10 days |
106 CFU g−1 |
EP Fec, GSI, and Ovolution rate ↑; Oocyte maturation G and FD ↑; |
[29][31][107][117][29,31,176,185] |
| Oocyte maturation FD and FM ↑ |
[100][170] |
| Follicular survival ↑ and apoptosis ↓ |
[30] |
| Lab. rhamnosus IMC 501 |
D. rerio |
40 males and females |
10 days |
106 CFU g−1 |
Embryo development ↑; HR ↑ |
[69][134] |
| Pediococcus acidilactici (Bactocell®) |
D. rerio |
5 wild males |
10 days |
106 CFU g−1 |
SP testicular cells ↑ |
[118][164] |
| Lab. rhamnosus CECT8361 and Bifidobacterium longum CECT7347 |
D. rerio |
36 Males |
21 days |
109 CFU g−1 |
SP SQ, SDn, SM ↑ |
[114][182] |
| PrimaLac® (Lab. acidophilus, Lab. casei, Enterococcus faecium, Bifidobacterium thermophilum) |
X. helleri |
10 females and 3 males |
182 days |
0.04%, 0.09% and 0.14% |
EP Fec and GSI ↑; SR (fry) ↑ |
[28] |
| P. acidilactici |
Carassius auratus (Goldfish) |
720 fishes |
180 days |
0.1, 0.2, and 0.3% |
EP GSI, HSI, AF, RF, ED, OD, FR, and HR ↑; SP SM, SD, SDn, and Stc ↑ |
[68][133] |
| Lab. rhamnosus IMC 501 |
Fundulus heteroclitus (Killifish) |
10 females and 10 males |
8 days |
106 CFU mL−1 |
EP GSI, Fec ↑ and HR →; SR (fry) ↑; GP L and W ↑ |
[36] |