You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by 红 晓.
胰岛素受体(The insulin receptor (IR)是一种跨膜蛋白,由胰岛素信号通路中的配体激活。IR被认为是临床干预的新型治疗靶点,考虑到其蛋白质和A-亚型在多种癌症,阿尔茨海默病和人类2型糖尿病中的过表达。同时,由于它对昆虫的多种生理影响,它也可能成为害虫管理的潜在目标。) is a transmembrane protein that is activated by ligands in insulin signaling pathways. The IR has been considered as a novel therapeutic target for clinical intervention, considering the overexpression of its protein and A-isoform in multiple cancers, Alzheimer’s disease, and Type 2 diabetes mellitus in humans. Meanwhile, it may also serve as a potential target in pest management due to its multiple physiological influences in insects.
- insulin receptor
- function
- agonists
- antagonists
- mechanism
1. Introduction
The insulin receptor (IR) is a transmembrane protein and part of the tyrosine kinase receptors (RTK). It exists as covalently bound receptor dimers at the cell surface [1]. The IR plays essential roles in metabolism, cell growth, and development by transmitting the binding of extracellular ligands into several intracellular signaling cascades [2,3,4][2][3][4]. Previous studies have demonstrated that ligands and the insulin signaling IR are highly conserved among human beings and insects [5,6,7][5][6][7].
In human beings, the function of the IR has been studied for many years, and it has been found to play a crucial role in multiple chronic diseases, including Alzheimer’s disease (AD) [8], Type 2 diabetes mellitus (T2DM) [9[9][10],10], and various cancers [2[2][11][12][13],11,12,13], as well as neurodegenerative disorders [14] and metabolic syndromes [15]. For T2DM, the destruction and dysfunction of pancreatic β-cells are common occurrences, and insulin injection is the only choice for glycemic control [16]. The dramatic increase in T2DM over the globe has led to increasing requirements for insulin. Moreover, insulin injection may require more than one shot each day, is hazardous and inconvenient, causes tissue irritation, abscesses, discomfort, etc., and local allergic reactions, lipoatrophy, lipohypertrophy, etc., are common complications of subcutaneous injections [17,18][17][18]. Because of the multiple problems associated with insulin injection, orally active insulin-mimetic compounds would be an ideal substitute [19]. For cancer, IR makes an attractive anticancer target owing to its overexpression in a variety of cancers, especially prostate and breast cancers [20]. Therefore, regulators of the IR, such as β-site amyloid precursor protein cleaving enzyme 1 (BACE1), have been regarded as potential therapeutic target [20,21][20][21]. Similarly, IR modulators such as ceritinib and anti-idiotypic antibody AK98 (an off-target IR inhibitor) have been suggested as promising drugs for the treatment of brain tumors and breast cancer, respectively [22,23][22][23].
In insects, current evidence points to the roles of the IR in regulating development, reproduction, lifespan, caste differentiation, and wing polyphenism [24,25,26][24][25][26]. To our knowlNedge, neonicotinoid insecticides (e.g., imidacloprid) are selective agonists of the nicotinic acetylcholine receptor (nAChR) that have been widely used to control various insects [27]. Likewise, ryanodine and diamides are commercial insecticides that are antagonists or activators of insect ryanodine receptors (RyRs) [28]. It may be deduced that modulators of the IR that selectively activate or inhibit the IR may be of considerable value in providing promising drugs for the control of human disease, or as insecticides for the control of insects [29]. In this regard, medicines specifically targeting the IR are diverse. However, IR-targeting insecticides are still lacking. Owing to the persistent use of traditional synthetic insecticides, insect resistance has become increasingly serious. Therefore, there is a growing need for new insecticides with new mechanisms of action. Thus IR-targeting insecticides represent an opportunity in the research and development of insecticides.
2. Biology Studies of the IR
2.1. Molecular Structure of the IR
Biochemically, the IR is encoded by a single gene. The coding region of the IR gene has 22 exons and 21 introns [30]. The alternative splicing of exon 11 encodes a 12-amino-acid sequence at the C-terminus of the α-subunit of the IR gene during transcription, resulting in the formation of the isoforms IR-A and IR-B [31]. IR-B is a mature isoform due to the fact that it includes the 12-amino-acid sequence, while the fetal isoform IR-A does not [10,32][10][32]. Both isoforms are expressed in most of the cells associated with energy homeostasis, such as adipocytes, hepatocytes, myocytes, and placenta vascular endothelium; however, they present different functional features [10,33][10][33]. Several in vitro and in vivo studies have confirmed that the expression and response of the two isoforms are different in breast cancer and T2DM [11]. IR-B possesses important metabolic functions and is the dominant isoform [2]. Conversely, the less-differentiated isoform IR-A is principally expressed in cancer cells [32]. Activation of IR-A promotes the growth of the cancer cells [34]. IR structural studies have previously been described in detail [35,36,37,38][35][36][37][38] (Table 1). The IR is a glycosylated, disulfide-linked (αβ)2 transmembrane homodimer consisting of two repeated ectodomains (ECD), a single transmembrane helix, and two intracellular cytoplasmic domain that includes a tyrosine kinase domain (TKD) (Figure 1a) [38,39][38][39]. The α-subunit constitutes most of the IR-ECD, while the β-subunit is necessary for the IR-ECD, the transmembrane domain (TMD), and the intracellular TKD [38].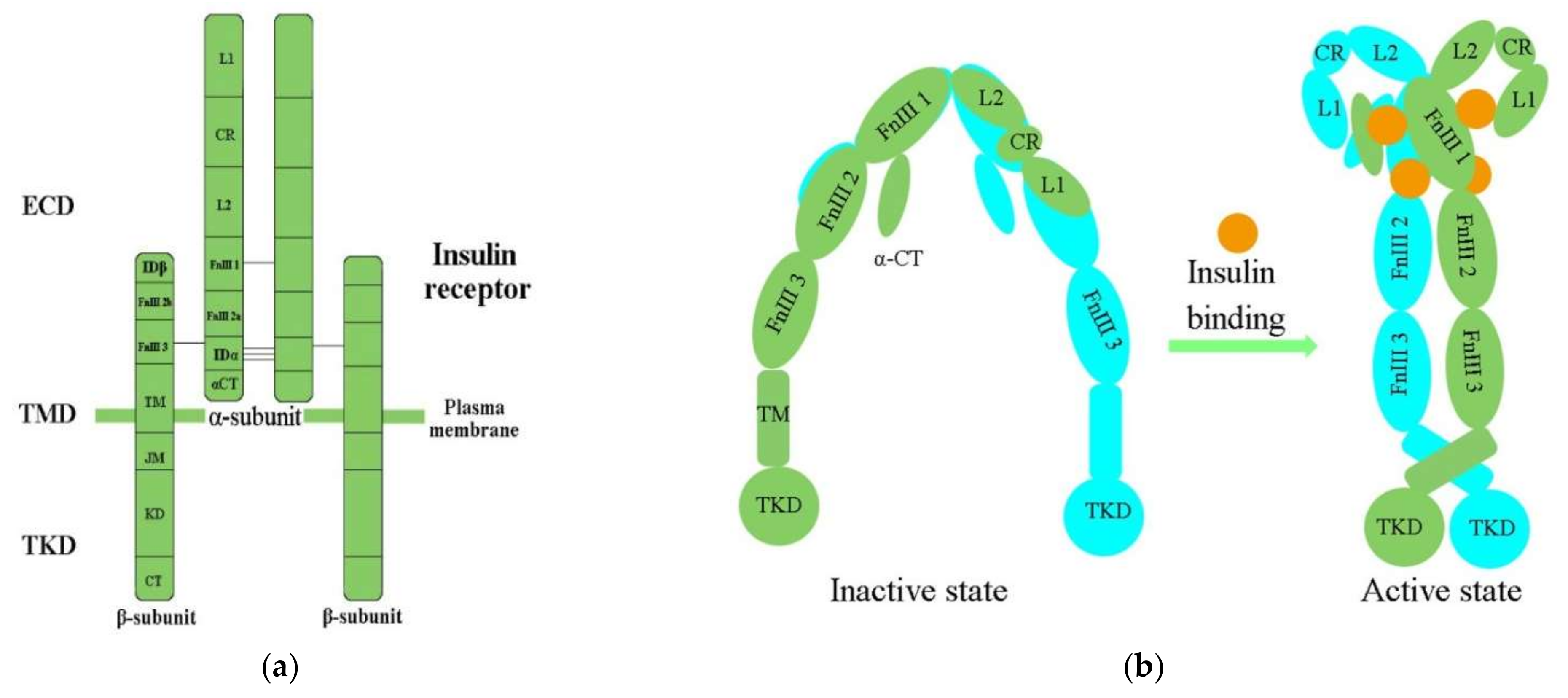
Figure 1. (a) The architectural domain of the IR (αβ) 2 homodimer. Black lines indicate the intersubunit disulfide bonds; (b) Inactive and active states of the IR; L1, L2, leucine-rich repeat domains 1, 2; CR, cysteine-rich domain; FnIII-1, 2, 3, fibronectin type-III domains 1, 2, 3; αCT, α C-terminal regions; TM, transmembrane; JM, juxtamembrane; KD, kinase domain; CT, C-terminal tail; ECD, ectodomain; TMD, transmembrane domain; TKD, tyrosine kinase domain.
Table 1. Summary of the available structures of IR.
| Classification | Structure of IR | References |
|---|---|---|
| Domain layout | an (αβ)2 disulfide-linked homodimer | [35] |
| cDNA sequenced | α chain lies on the N-terminal of the β chain | |
| 3D structure of human apo IR ectodomain | intracellular unphosphorylated from TKD (2.1 Å resolution, PDB 1IRK) | |
| receptor’s isolated L1-CR-L2 module (2.32 Å resolution, PDB 2HR7) | ||
| intact receptor ectodomain in apo form (3.8 Å resolution, PDB 2DTG) | ||
| CryoEM structures of IR | insulin holoreceptor (full-length receptor inclusive of transmembrane and cytoplasmic elements) | [42][40] |
| isolated receptor ectodomain | [41,43][41][42] | |
| an ectodomain construct (leucine-zippered receptor ectodomain) | [44][43] |
2.2. Activation of the IR
Physiologically, the function of the IR is activated in the insulin/IGF-1-like signal (IIS) pathway by the ligand [2]. The IIS pathway is commonly known as a significant nutrient-dependent endocrine pathway and regulates numerous physiological processes, such as metabolism, growth and development, and so on [6]. In the IIS pathway, the IR regulates two primary cell-signaling cascades (Figure 2) [53]: the phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway and the mitogen-activated protein kinase (MAPK) pathway (extracellular-signal regulated kinase signaling pathway (ERK)) [53,54,55,56][53][54][55][56]. The PI3K/AKT pathway is primarily responsible for controlling metabolic processes such as glucose transportation and the synthesis of lipids, proteins, and glycogen. In contrast, the MAPK pathway is primarily related to the mitogenic effects of insulin and is mainly responsible for cell growth and proliferation [56,57][56][57].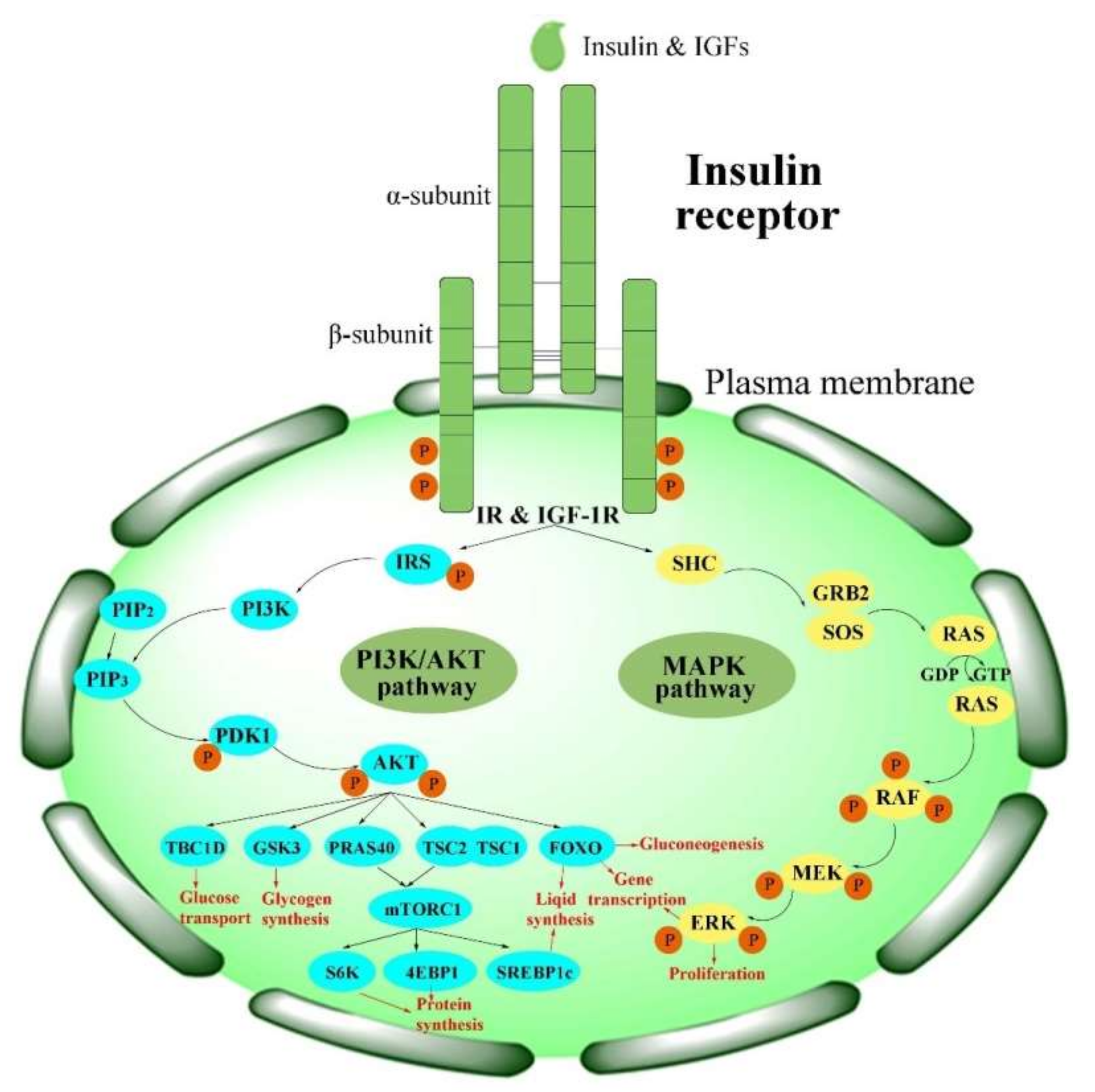
Figure 2. Activation of the IR in insulin signaling pathways. PI3K/AKT pathways: phosphatidylinositol-3-kinase signaling pathways; MAPK pathway: mitogen-activated protein kinase pathway.
3. Functions of the IR
3.1. The Functions of the IR in Human Beings
3.1. The Functions of the IR in Human Beings
在人类中,In humans, the IR在全身营养稳态和各种疾病中起着至关重要的作用,例如 plays a crucial role in whole-body nutrient homeostasis and in various diseases, such as AD [8],[8], T2DM [4,9,10],肥胖[70],动脉粥样硬化[31],多发性癌症[11,12,13]和心血管疾病[71],以及神经退行性疾病[14],代谢综合征[15]和多囊卵巢综合征[72]][4][9][10], obesity [70], atherosclerosis [31], multiple cancers [11][12][13], and cardiovascular disease [71], as well as neurodegenerative disorders [14], metabolic syndrome [15] and polycystic ovary syndrome [72].因此,有必要了解 Thus, it is necessary to understand the cellular expression and the functions of the IR的细胞表达和功能,以便提出新的治疗概念并开发新药。 in order to propose new treatment concepts and to develop novel drugs. The IR介导全身营养稳态,并通过肝脏、肌肉和脂肪组织中经典的胰岛素反应靶标普遍表达[3]。最近的研究表明, mediates whole-body nutrient homeostasis and is expressed ubiquitously through the classic insulin-responsive targets in the liver, muscle, and adipose tissue [3]. A has demonstrated that the IR分布在活海马脑神经元的树突状轴和棘中[73]。 is distributed in both dendritic shafts and spines in living hippocampal brain neurons [73]. Knockout of the IR的敲除导致许多靶器官受损。 resulted in many impaired target organs. Hepatic deletion of the IR的肝脏缺失导致高血糖、脂肪酸代谢紊乱以及脂肪酸氧化酶表达增加[74]。在粘膜上皮细胞中, led to hyperglycemia, disorders in fatty acid metabolism, and an increase in the expression of fatty acid oxidation enzymes [74]. In mucosal epithelial cells, the IR与线粒体中的电压依赖性阴离子通道 interacts with the voltage-dependent anion channel-1(VDAC1)相互作用。IR基因的敲除触发了强大的线粒体碎片化和极化改变[75],而 (VDAC1) in mitochondria. Knockdown of the IR gene triggered robust mitochondrial fragmentation and altered polarization [75], while knockout of the β细胞-cell IR基因的敲除导致胰岛素分泌受损[76]。此外, gene led to impaired insulin secretion [76]. Additionally, missense mutations of the IR的错义突变可能导致严重的遗传性胰岛素抵抗综合征[77]。 may cause severe inherited insulin resistance syndromes [77]. The IR是易位到细胞核的细胞表面受体,与染色质中的RNA聚合酶II密切相关[78]。在细胞中,宿主细胞因子 is a cell-surface receptor translocating to the nucleus, and is associated strongly with RNA polymerase II in the chromatin [78]. In the cell, host cell factor-1( (HCF-1)充当转录联合调节剂,功能性地介导IR与位于基因启动子中特定位点的结合[79]。) acts as a transcriptional coregulator functionally mediating the binding of the IR to specific sites located in the gene promoters [79]. HCF-1介导 mediates the association between the IR和DNA之间的关联。HCF-1通过DNA序列特异性转录因子间接结合DNA,然后在染色质中与IR和 and DNA. HCF-1 binds to DNA indirectly through DNA sequence-specific transcription factors, and then forms a complex with the IR and Thanatos相关的蛋白质结构域蛋白11(-associated protein domain-containing protein 11 (THAP11)形成复合物。HCF-1的敲低可抑制) in the chromatin. Knockdown of HCF-1 can inhibit the binding ability of the IR to the promotors [79]. Another study indicated that the mRNA and protein levels of the IR与启动子的结合能力[79]。另一项研究表明,妊娠期糖尿病( were obviously reduced in the subcutaneous and visceral adipose tissue of women with gestational diabetes mellitus (GDM)女性皮下和内脏脂肪组织中s) [80]. The decrease in IR的 mRNA和蛋白质水平明显降低[80]。 was accompanied by a decrease in methylation levels of the IR promRNA的降低伴随着oter [80]. This phenomenon has also been observed in the hypothalamus [81]. The methylation degree of the IR启动子甲基化水平的降低[80]。在下丘脑中也观察到这种现象[81]。 nuclear factor IR启动子内 (IR核因子I(IRNF-I)结合位点的甲基化程度与IR的基因水平呈显着负相关。这些发现为进一步研究IR的功能和机制开辟了新的途径。需要更多研究来证明IR序列中的表观遗传修饰是否会影响IR的IR表达[82]。NF-I) binding site within the IR promoter was dramatically inversely correlated with the gene level of the IR. These findings have opened a new avenue for further studies on the functions and mechanisms of the IR. More studies focusing on demonstrating whether epigenetic modifications in the IR sequence impact IR expression of the IR are needed [82].3.2. The Functions of the IR in Insects
3.2. 昆虫中红外线的功能
对人类红外的研究引起了人们对昆虫中红外功能的兴趣,以及随之而来的开发具有高效率和低毒性的新型红外靶向杀虫剂的可能性。Studies of the IR in human beings have raised interest in the functions of the IR in insects and the consequent possibility for the development of new IR-targeting insecticides with high efficiency and low toxicity. 在昆虫中,已经揭示了In insects, multiple functions of the IR的多种功能[83]。众所周知, have been revealed [83]. The IR与胚胎后发育[84,85],营养表型可塑性和体型控制[86,87],生殖和透析[55],生殖和重生[55]以及昼夜节律和行为[88,89,90]有关,直接或通过与其他主要激素(如幼年激素( is well-known to be implicated—either directly or by crosstalk with other major hormones such as juvenile hormone (JH)和蜕皮甾醇(尤其是) and ecdysteroids (especially 20-羟基依蔀酮,20E)相联系[84,85],基于营养的表型可塑性和体型控制[86,87],繁殖和透析[55],以及昼夜节律和行为[88,89,90]]hydroxyecdysone, 20E)—in post-embryonic development [84][85], nutrition-based phenotypic plasticity and body size control [86][87], reproduction and diapause [55], and circadian rhythmicity and behaviors [88][89][90]. The IR在昆虫光周期、寿命和衰老中也是必不可少的,因为它与新陈代谢和生长有关[25,91,92,93]。总体而言,研究表明, is also indispensable in insect photoperiodism, lifespan, and aging due to its relation to metabolism and growth [25][91][92][93]. Overall, studies have indicated that the IR在昆虫生长[94],发育和繁殖[95,96],多态性[24],寿命[97]和产卵[98]中是不可或缺的。因此, is indispensable in insect growth [94], development and reproduction [95][96], polymorphism [24], lifespan [97], and oviposition [98]. Therefore, the IR是害虫和寄生虫管理的重要目标。 represents an important target for the management of pests and parasites.References
- Ward, C.W.; Lawrence, M.C. Ligand-induced activation of the insulin receptor: A multi-step process involving structural changes in both the ligand and the receptor. Bioessays 2009, 31, 422–434.
- Vigneri, R.; Goldfine, I.D.; Frittitta, L. Insulin, insulin receptors, and cancer. J. Endocrinol. Investig. 2016, 39, 1365–1376.
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44.
- Yunn, N.O.; Kim, J.; Kim, Y.; Leibiger, I.; Berggren, P.O.; Ryu, S.H. Mechanistic understanding of insulin receptor modulation: Implications for the development of anti-diabetic drugs. Pharmacolo. Therapeut. 2018, 185, 86–98.
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221.
- Lin, X.; Smagghe, G. Roles of the insulin signaling pathway in insect development and organ growth. Peptides 2019, 122, 169923.
- Liu, C.Y.; Zhao, W.L.; Wang, J.X.; Zhao, X.F. Cyclin-dependent kinase regulatory subunit 1 promotes cell proliferation by insulin regulation. Cell Cycle 2015, 14, 3045–3057.
- Craft, S.; Cholerton, B.; Baker, L.D. Insulin and Alzheimer’s disease: Untangling the web. J. Alzheimer’s Dis. 2013, 33 (Suppl. S1), S263–S275.
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814.
- Westermeier, F.; Saez, T.; Arroyo, P.; Toledo, F.; Gutierrez, J.; Sanhueza, C.; Pardo, F.; Leiva, A.; Sobrevia, L. Insulin receptor isoforms: An integrated view focused on gestational diabetes mellitus. Diabetes Metab. Res. Rev. 2016, 32, 350–365.
- Vella, V.; Milluzzo, A.; Scalisi, N.; Vigneri, P.; Sciacca, L. Insulin receptor isoforms in cancer. Int. J. Mol. Sci. 2018, 19, 3615.
- Sciacca, L.; Le Moli, R.; Vigneri, R. Insulin analogs and cancer. Front. Endocrinol. 2012, 3, 21.
- Malaguarnera, R.; Belfiore, A. The insulin receptor: A new target for cancer therapy. Front. Endocrinol. 2011, 2, 93.
- Stoeckel, L.E.; Arvanitakis, Z.; Gandy, S.; Small, D.; Kahn, C.R.; Pascual-Leone, A.; Pawlyk, A.; Sherwin, R.; Smith, P. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res 2016, 5, 353.
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017, 28, 497–505.
- Campbell, R.K. Type 2 diabetes: Where we are today: An overview of disease burden, current treatments, and treatment strategies. J. Am. Pharm. Assoc. 2009, 49, S3–S9.
- Ermakova, A.; Stauffer, M.E.; Sieradzan, R.; Taylor, S. Characterizing the clinical and economic burden of type 2 diabetes (T2DM) patients on multiple daily injections (MDI) of insulin: A systematic literature review. Value Health 2018, 21, S70.
- Buyruk, B.A.; Kebapci, N.; Yorulmaz, G.; Alaguney, E.S.; Akalin, A.; Efe, B. Prevalence and risk factors of lipohypertrophy and lipoatrophy in diabetes patients receiving insulin therapy. Diabetes 2019, 68, 59.
- Brietzke, S.A. Oral antihyperglycemic treatment options for type 2 diabetes mellitus. Med. Clin. North Am. 2015, 99, 87–106.
- Singh, P.; Alex, J.M.; Bast, F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: Novel treatment strategies for cancer. Med. Oncol. 2014, 31, 805.
- Meakin, P.J.; Mezzapesa, A.; Benabou, E.; Haas, M.E.; Bonardo, B.; Grino, M.; Brunel, J.M.; Desbois-Mouthon, C.; Biddinger, S.B.; Govers, R.; et al. The beta secretase BACE1 regulates the expression of insulin receptor in the liver. Nat. Commun. 2018, 9, 1306.
- Russo, A.; Paret, C.; Alt, F.; Burhenne, J.; Fresnais, M.; Wagner, W.; Glaser, M.; Bender, H.; Huprich, S.; Harter, P.N.; et al. Ceritinib-induced regression of an insulin-like growth factor-driven neuroepithelial brain tumor. Int. J. Mol. Sci. 2019, 20, 4267.
- Wenbin, K.; Xiaoqin, L.; Qiuchan, D.; Xinwen, Z.; Xiaoqin, X.; Fangyuan, S.; Dabao, H.; Shuangjiu, Z. Development of a novel insulin receptor (IR) antagonist that exhibits anti-breast tumor activity. Human Cell 2020, 33, 1204–1217.
- Lin, X.; Lavine, L.C. Endocrine regulation of a dispersal polymorphism in winged insects: A short review. ScienceDirect 2018, 25, 20–24.
- Guo, S.; Wang, X.; Kang, L. Special significance of non-Drosophila insects in aging. Front. Cell Dev. Biol. 2020, 8, 576571.
- Alvarez-Rendon, J.P.; Salceda, R.; Riesgo-Escovar, J.R. Drosophila melanogaster as a model for diabetes type 2 progression. Biomed. Res. Int. 2018, 2018, 1417528.
- Houchat, J.N.; Cartereau, A.; Le Mauff, A.; Taillebois, E.; Thany, S.H. An overview on the effect of neonicotinoid insecticides on mammalian cholinergic functions through the activation of neuronal nicotinic acetylcholine receptors. Int. J. Environ. Res. Public Health 2020, 17, 3222.
- Sun, Z.; Xu, H. Ryanodine receptors for drugs and insecticides: An overview. Mini. Rev. Med. Chem. 2019, 19, 22–33.
- Sun, Z.; Lv, M.; Huang, W.; Li, T.; Xu, H. Development of botanical pesticides: Exploration on the phenotype of vestigial wings of insect pests induced by plant natural products or their derivatives by blocking tyrosine phosphorylation of insulin receptor 1. J. Agric. Food Chem. 2022, 70, 2117–2126.
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009, 30, 586–623.
- Beneit, N.; Fernandez-Garcia, C.E.; Martin-Ventura, J.L.; Perdomo, L.; Escribano, O.; Michel, J.B.; Garcia-Gomez, G.; Fernandez, S.; Diaz-Castroverde, S.; Egido, J.; et al. Expression of insulin receptor (IR) A and B isoforms, IGF-IR, and IR/IGF-IR hybrid receptors in vascular smooth muscle cells and their role in cell migration in atherosclerosis. Cardiovasc. Diabetol. 2016, 15, 161.
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin receptor isoforms in physiology and disease: An updated view. Endocr. Rev. 2017, 38, 379–431.
- Huang, J.; Morehouse, C.; Streicher, K.; Higgs, B.W.; Gao, J.; Czapiga, M.; Boutrin, A.; Zhu, W.; Brohawn, P.; Chang, Y.; et al. Altered expression of insulin receptor isoforms in breast cancer. PLoS ONE. 2011, 6, e26177.
- Heidegger, I.; Kern, J.; Ofer, P.; Klocker, H.; Massoner, P. Oncogenic functions of IGF1R and INSR in prostate cancer include enhanced tumor growth, cell migration and angiogenesis. Oncotarget 2014, 5, 2723–2735.
- Lawrence, M.C. Understanding insulin and its receptor from their three-dimensional structures. Mol Metab. 2021, 52, 101255.
- De Meyts, P. Insulin/receptor binding: The last piece of the puzzle? What recent progress on the structure of the insulin/receptor complex tells us (or not) about negative cooperativity and activation. Bioessays 2015, 37, 389–397.
- Croll, T.I.; Smith, B.J.; Margetts, M.B.; Whittaker, J.; Weiss, M.A.; Ward, C.W.; Lawrence, M.C. Higher-resolution structure of the human insulin receptor ectodomain: Multi-modal inclusion of the insert domain. Structure 2016, 24, 469–476.
- Ye, L.; Maji, S.; Sanghera, N.; Gopalasingam, P.; Gorbunov, E.; Tarasov, S.; Epstein, O.; Klein-Seetharaman, J. Structure and dynamics of the insulin receptor: Implications for receptor activation and drug discovery. Drug Discov. Today 2017, 22, 1092–1102.
- Scapin, G.; Dandey, V.P.; Zhang, Z.; Prosise, W.; Hruza, A.; Kelly, T.; Mayhood, T.; Strickland, C.; Potter, C.S.; Carragher, B. Structure of the insulin receptor-insulin complex by single-particle cryo-EM analysis. Nature 2018, 556, 122–125.
- Uchikawa, E.; Choi, E.; Shang, G.; Yu, H.; Bai, X.C. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife 2019, 8, e48630.
- Gutmann, T.; Kim, K.H.; Grzybek, M.; Walz, T.; Coskun, U. Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. J. Cell Biol. 2018, 217, 1643–1649.
- Gutmann, T.; Schafer, I.B.; Poojari, C.; Brankatschk, B.; Vattulainen, I.; Strauss, M.; Coskun, U. Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain. J. Cell Biol. 2020, 219, e201907210.
- Weis, F.; Menting, J.G.; Margetts, M.B.; Chan, S.J.; Xu, Y.; Tennagels, N.; Wohlfart, P.; Langer, T.; Muller, C.W.; Dreyer, M.K.; et al. The signalling conformation of the insulin receptor ectodomain. Nat. Commun. 2018, 9, 4420.
- McKern, N.M.; Lawrence, M.C.; Streltsov, V.A.; Lou, M.Z.; Adams, T.E.; Lovrecz, G.O.; Elleman, T.C.; Richards, K.M.; Bentley, J.D.; Pilling, P.A.; et al. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature 2006, 443, 218–221.
- Whittaker, J.; Whittaker, L. Characterization of the functional insulin binding epitopes of the full-length insulin receptor. J. Biol. Chem. 2005, 280, 20932–20936.
- Menting, J.G.; Whittaker, J.; Margetts, M.B.; Whittaker, L.J.; Kong, G.K.; Smith, B.J.; Watson, C.J.; Zakova, L.; Kletvikova, E.; Jiracek, J.; et al. How insulin engages its primary binding site on the insulin receptor. Nature 2013, 493, 241–245.
- Chrudinova, M.; Zakova, L.; Marek, A.; Socha, O.; Budesinsky, M.; Hubalek, M.; Picha, J.; Machackova, K.; Jiracek, J.; Selicharova, I. A versatile insulin analog with high potency for both insulin and insulin-like growth factor 1 receptors: Structural implications for receptor binding. J. Biol. Chem. 2018, 293, 16818–16829.
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 activates its receptor. Elife 2014, 3, e03772.
- Xu, Y.; Kong, G.K.; Menting, J.G.; Margetts, M.B.; Delaine, C.A.; Jenkin, L.M.; Kiselyov, V.V.; De Meyts, P.; Forbes, B.E.; Lawrence, M.C. How ligand binds to the type 1 insulin-like growth factor receptor. Nat. Commun. 2018, 9, 821.
- Yunn, N.-O.; Park, M.; Noh, J.; Ryu, S.H. Stepwise autophosphorylation regulates biased agonism of the insulin receptor. Faseb. J. 2019, 34, s1.08794.
- Hernandez-Sanchez, C.; Mansilla, A.; Pablo, F.d.; Zardoya, R. Evolution of the insulin receptor family and receptor isoform expression in vertebrates. Mol. Biol. Evol. 2008, 25, 1043–1053.
- Li, J.; Park, J.; Mayer, J.P.; Webb, K.J.; Uchikawa, E.; Wu, J.Y.; Liu, S.; Zhang, X.W.; Stowell, M.H.B.; Choi, E.; et al. Synergistic activation of the insulin receptor via two distinct sites. Nat. Struct. Mol. Biol. 2022, 29, 357–368.
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459.
- Lawrence, M.C.; McKern, N.M.; Ward, C.W. Insulin receptor structure and its implications for the IGF-1 receptor. Curr. Opin. Struct Biol. 2007, 17, 699–705.
- Sim, C.; Denlinger, D.L. Insulin signaling and the regulation of insect diapause. Front. Physiol. 2013, 4, 189.
- Hall, C.; Yu, H.; Choi, E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp. Mol. Med. 2020, 52, 911–920.
- Cai, W.; Sakaguchi, M.; Kleinridders, A.; Gonzalez-Del Pino, G.; Dreyfuss, J.M.; O’Neill, B.T.; Ramirez, A.K.; Pan, H.; Winnay, J.N.; Boucher, J.; et al. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat. Commun. 2017, 8, 14892.
- Okada, Y.; Katsuki, M.; Okamoto, N.; Fujioka, H.; Okada, K. A specific type of insulin-like peptide regulates the conditional growth of a beetle weapon. PLoS Biol. 2019, 17, e3000541.
- Laustsen, P.G.; Russell, S.J.; Cui, L.; Entingh-Pearsall, A.; Holzenberger, M.; Liao, R.; Kahn, C.R. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol. Cell Biol. 2007, 27, 1649–1664.
- Nassel, D.R.; Vanden Broeck, J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol. Life Sci. 2016, 73, 271–290.
- Inoue, H. Central insulin-mediated regulation of hepatic glucose production. Endocr. J. 2016, 63, EJ15-0540.
- Nijhout, H.F.; Smith, W.A.; Schachar, I.; Subramanian, S.; Tobler, A.; Grunert, L.W. The control of growth and differentiation of the wing imaginal disks of Manduca sexta. Dev. Biol. 2007, 302, 569–576.
- Chen, C.H.; Pan, J.; Di, Y.Q.; Liu, W.; Hou, L.; Wang, J.X.; Zhao, X.F. Protein kinase C delta phosphorylates ecdysone receptor B1 to promote gene expression and apoptosis under 20-hydroxyecdysone regulation. Proc. Natl. Acad. Sci. USA 2017, 114, E7121–E7130.
- Ajaha, A.; Bouayad, N.; Aarab, A.; Rharrabe, K. Effect of 20-hydroxyecdysone, a phytoecdysteroid, on development, digestive, and detoxification enzyme activities of Tribolium castaneum (Coleoptera: Tenebrionidae). J. Insect Sci. 2019, 19, 18.
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006, 51, 1–24.
- Nagasawa, H.; Kataoka, H.; Isogai, A.; Tamura, S.; Suzuki, A.; Ishizaki, H.; Mizoguchi, A.; Fujiwara, Y.; Suzuki, A. Amino-terminal amino acid sequence of the silkworm prothoracicotropic hormone: Homology with insulin. Science 1984, 226, 1344–1345.
- Siddle, K. Molecular basis of signaling specificity of insulin and IGF receptors: Neglected corners and recent advances. Front. Endocrinol. 2012, 3, 34.
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014, 6, a009191.
- Versteyhe, S.; Blanquart, C.; Hampe, C.; Mahmood, S.; Christeff, N.; Meyts1, P.D.; Gray, S.G.; Issad, T. Insulin receptor substrates-5 and -6 are poor substrates for the insulin receptor. Mol. Med. Rep. 2010, 3, 189–193.
- Schmidt, V.; Schulz, N.; Yan, X.; Schurmann, A.; Kempa, S.; Kern, M.; Bluher, M.; Poy, M.N.; Olivecrona, G.; Willnow, T.E. SORLA facilitates insulin receptor signaling in adipocytes and exacerbates obesity. J. Clin. Investig. 2016, 126, 2706–2720.
- Rask-Madsen, C.; Kahn, C.R. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2052–2059.
- Shi, X.; Xie, X.; Jia, Y.; Li, S. Associations of insulin receptor and insulin receptor substrates genetic polymorphisms with polycystic ovary syndrome: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2016, 42, 844–854.
- Gralle, M.; Labrecque, S.; Salesse, C.; De Koninck, P. Spatial dynamics of the insulin receptor in living neurons. J. Neurochem. 2020, 156, 88–105.
- Ling, A.V.; Gearing, M.E.; Semova, I.; Shin, D.J.; Clements, R.; Lai, Z.W.; Biddinger, S.B. FoxO1 is required for most of the metabolic and hormonal perturbations produced by hepatic insulin receptor deletion in male mice. Endocrinology 2018, 159, 1253–1263.
- Titone, R.; Robertson, D.M. Insulin receptor preserves mitochondrial function by binding VDAC1 in insulin insensitive mucosal epithelial cells. FASEB J. 2019, 34, 754–775.
- Oakie, A.; Zhou, L.; Rivers, S.; Cheung, C.; Li, J.; Wang, R. Postnatal knockout of beta cell insulin receptor impaired insulin secretion in male mice exposed to high-fat diet stress. Mol. Cell Endocrinol. 2020, 499, 110588.
- Ardon, O.; Procter, M.; Tvrdik, T.; Longo, N.; Mao, R. Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol. Genet. Metab. Rep. 2014, 1, 71–84.
- Greenhill, C. Insulin and the insulin receptor regulate gene expression. Nat. Rev. Endocrinol. 2019, 15, 315.
- Hancock, M.L.; Meyer, R.C.; Mistry, M.; Khetani, R.S.; Wagschal, A.; Shin, T.; Ho Sui, S.J.; Naar, A.M.; Flanagan, J.G. Insulin receptor associates with promoters genome-wide and regulates gene expression. Cell 2019, 177, 722–736.
- Ott, R.; Melchior, K.; Stupin, J.H.; Ziska, T.; Schellong, K.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Reduced insulin receptor expression and altered DNA methylation in fat tissues and blood of women with GDM and offspring. J. Clin. Endocrinol. Metab. 2019, 104, 137–149.
- Schellong, K.; Melchior, K.; Ziska, T.; Ott, R.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Hypothalamic insulin receptor expression and DNA promoter methylation are sex-specifically altered in adult offspring of high-fat diet (HFD)-overfed mother rats. J. Nutr. Biochem. 2019, 67, 28–35.
- Stolzenbach, F.; Valdivia, S.; Ojeda-Provoste, P.; Toledo, F.; Sobrevia, L.; Kerr, B. DNA methylation changes in genes coding for leptin and insulin receptors during metabolic-altered pregnancies. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165465.
- Pan, J.; Di, Y.Q.; Li, Y.B.; Chen, C.H.; Wang, J.X.; Zhao, X.F. Insulin and 20-hydroxyecdysone oppose each other in the regulation of phosphoinositide-dependent kinase-1 expression during insect pupation. J. Biol. Chem. 2018, 293, 18613–18623.
- Liu, Y.; Zhou, S.; Ma, L.; Tian, L.; Wang, S.; Sheng, Z.; Jiang, R.J.; Bendena, W.G.; Li, S. Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 2010, 56, 1436–1444.
- Li, Y.L.; Yao, Y.X.; Zhao, Y.M.; Di, Y.Q.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone counteracts insulin signaling via insulin receptor dephosphorylation. J. Biol. Chem. 2021, 296, 100318.
- Emlen, D.J.; Warren, I.A.; Johns, A.; Dworkin, I.; Lavine, L.C. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 2012, 337, 860–864.
- Casasa, S.; Moczek, A.P. Insulin signalling’s role in mediating tissue-specific nutritional plasticity and robustness in the horn-polyphenic beetle Onthophagus taurus. Proc. Biol. Sci. 2018, 285, 20181631.
- Eichler, K.; Li, F.; Litwin-Kumar, A.; Park, Y.; Andrade, I.; Schneider-Mizell, C.M.; Saumweber, T.; Huser, A.; Eschbach, C.; Gerber, B.; et al. The complete connectome of a learning and memory centre in an insect brain. Nature 2017, 548, 175–182.
- Thum, A.S.; Gerber, B. Connectomics and function of a memory network: The mushroom body of larval Drosophila. Curr. Opin. Neurobiol. 2019, 54, 146–154.
- Eschment, M.; Franz, H.R.; Gullu, N.; Holscher, L.G.; Huh, K.E.; Widmann, A. Insulin signaling represents a gating mechanism between different memory phases in Drosophila larvae. PLoS Genet. 2020, 16, e1009064.
- Toprak, U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 2020, 11, 434.
- Smykal, V.; Pivarci, M.; Provaznik, J.; Bazalova, O.; Jedlicka, P.; Luksan, O.; Horak, A.; Vaneckova, H.; Benes, V.; Fiala, I.; et al. Complex evolution of insect insulin receptors and homologous decoy receptors, and functional significance of their multiplicity. Mol. Biol. Evol. 2020, 37, 1775–1789.
- Barbera, M.; Canas-Canas, R.; Martinez-Torres, D. Insulin-like peptides involved in photoperiodism in the aphid Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2019, 112, 103185.
- Lin, X.; Yu, N.; Smagghe, G. Insulin receptor regulates food intake through sulfakinin signaling in the red flour beetle, Tribolium castaneum. Peptides 2016, 80, 89–95.
- Sang, M.; Li, C.; Wu, W.; Li, B. Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene 2016, 585, 196–204.
- Nuss, A.B.; Brown, M.R.; Murty, U.S.; Gulia-Nuss, M. Insulin receptor knockdown blocks filarial parasite development and alters egg production in the southern house mosquito, Culex quinquefasciatus. PLoS Negl. Trop. Dis. 2018, 12, e0006413.
- Ihle, K.E.; Mutti, N.S.; Kaftanoglu, O.; Amdam, G.V. Insulin receptor substrate gene knockdown accelerates behavioural maturation and shortens lifespan in honeybee workers. Insects 2019, 10, 390.
- Han, B.; Zhang, T.; Feng, Y.; Liu, X.; Zhang, L.; Chen, H.; Zeng, F.; Wang, M.; Liu, C.; Li, Y.; et al. Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 2020, 123, 104049.
More
