In human beings, the function of the IR has been studied for many years, and it has been found to play a crucial role in multiple chronic diseases, including Alzheimer’s disease (AD)
[8], Type 2 diabetes mellitus (T2DM)
[9][10][9,10], and various cancers
[2][11][12][13][2,11,12,13], as well as neurodegenerative disorders
[14] and metabolic syndromes
[15]. For T2DM, the destruction and dysfunction of pancreatic β-cells are common occurrences, and insulin injection is the only choice for glycemic control
[16]. The dramatic increase in T2DM over the globe has led to increasing requirements for insulin. Moreover, insulin injection may require more than one shot each day, is hazardous and inconvenient, causes tissue irritation, abscesses, discomfort, etc., and local allergic reactions, lipoatrophy, lipohypertrophy, etc., are common complications of subcutaneous injections
[17][18][17,18]. Because of the multiple problems associated with insulin injection, orally active insulin-mimetic compounds would be an ideal substitute
[19]. For cancer, IR makes an attractive anticancer target owing to its overexpression in a variety of cancers, especially prostate and breast cancers
[20]. Therefore, regulators of the IR, such as β-site amyloid precursor protein cleaving enzyme 1 (BACE1), have been regarded as potential therapeutic target
[20][21][20,21]. Similarly, IR modulators such as ceritinib and anti-idiotypic antibody AK98 (an off-target IR inhibitor) have been suggested as promising drugs for the treatment of brain tumors and breast cancer, respectively
[22][23][22,23].
2. Biology Studies of the IR
2.1. Molecular Structure of the IR
Biochemically, the IR is encoded by a single gene. The coding region of the IR gene has 22 exons and 21 introns
[30]. The alternative splicing of exon 11 encodes a 12-amino-acid sequence at the C-terminus of the α-subunit of the IR gene during transcription, resulting in the formation of the isoforms IR-A and IR-B
[31]. IR-B is a mature isoform due to the fact that it includes the 12-amino-acid sequence, while the fetal isoform IR-A does not
[10][32][10,32]. Both isoforms are expressed in most of the cells associated with energy homeostasis, such as adipocytes, hepatocytes, myocytes, and placenta vascular endothelium; however, they present different functional features
[10][33][10,33]. Several in vitro and in vivo studies have confirmed that the expression and response of the two isoforms are different in breast cancer and T2DM
[11]. IR-B possesses important metabolic functions and is the dominant isoform
[2]. Conversely, the less-differentiated isoform IR-A is principally expressed in cancer cells
[32]. Activation of IR-A promotes the growth of the cancer cells
[34].
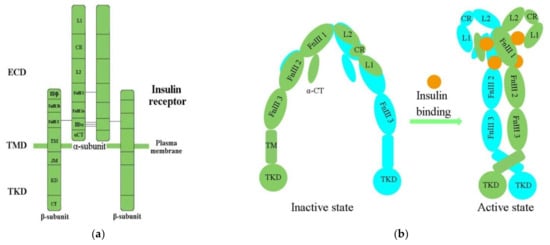
IR structural studies have previously been described in detail
[35][36][37][38][35,36,37,38] (
Table 1). The IR is a glycosylated, disulfide-linked (αβ)
2 transmembrane homodimer consisting of two repeated ectodomains (ECD), a single transmembrane helix, and two intracellular cytoplasmic domain that includes a tyrosine kinase domain (TKD) (
Figure 1a)
[38][39][38,39]. The α-subunit constitutes most of the IR-ECD, while the β-subunit is necessary for the IR-ECD, the transmembrane domain (TMD), and the intracellular TKD
[38].
Figure 1. (a) The architectural domain of the IR (αβ) 2 homodimer. Black lines indicate the intersubunit disulfide bonds; (b) Inactive and active states of the IR; L1, L2, leucine-rich repeat domains 1, 2; CR, cysteine-rich domain; FnIII-1, 2, 3, fibronectin type-III domains 1, 2, 3; αCT, α C-terminal regions; TM, transmembrane; JM, juxtamembrane; KD, kinase domain; CT, C-terminal tail; ECD, ectodomain; TMD, transmembrane domain; TKD, tyrosine kinase domain.
Table 1. Summary of the available structures of IR.
| Classification |
Structure of IR |
References |
| Domain layout |
an (αβ)2 disulfide-linked homodimer |
[35] |
| cDNA sequenced |
α chain lies on the N-terminal of the β chain |
| 3D structure of human apo IR ectodomain |
intracellular unphosphorylated from TKD (2.1 Å resolution, PDB 1IRK) |
| receptor’s isolated L1-CR-L2 module (2.32 Å resolution, PDB 2HR7) |
| intact receptor ectodomain in apo form (3.8 Å resolution, PDB 2DTG) |
| CryoEM structures of IR |
insulin holoreceptor (full-length receptor inclusive of transmembrane and cytoplasmic elements) |
[40][42] |
| isolated receptor ectodomain |
[41][42][41,43] |
| an ectodomain construct (leucine-zippered receptor ectodomain) |
[43][44] |
Determination of the three-dimensional (3D) crystal structure of the insulin-free IR-ECD through crystallography has revealed that the IR-ECD dimer roughly displays an inverted “U”- or “V”-shaped architecture
[37][44][37,40]. Specifically, L1 and CR together with L2 form one leg, while the linearly arranged FnIII domains form the other leg
[37]. However, the modular organization of the ECD, with high intrinsic flexibility and its complex ligand-binding properties, poses a challenge for structural studies of the IR. Furthermore, single-particle cryo-electron microscopy (cryo-EM) has revealed that the IR-ECD dimer converts the overall architecture from an autoinhibited inverted “V” shape into a “T”-shaped conformation, which was stabilized after binding insulin molecules to the N-terminal domains (
Figure 1b)
[39][40][41][39,41,42]. The L1, CR, and L2 domains of both IR promoters constitute the “T” horizontal part, while the FnIII-1, -2, and -3 domains of the IR dimer constitute the vertical piece of the “T”
[40][42].
Previous biochemical and mutagenesis models of insulin binding have identified two distinct binding sites on both the IR and insulin, termed site 1 (S1) and site 2 (S2)
[36]. The L1 subdomain and the α-CT helix residue have been confirmed to represent IR S1 site (IR-S1)
[39][45][46][39,45,46]. Evidence has indicated that IR-S1 is indispensable for insulin binding, and minor modifications of it were sufficient to change the IR’s specificity for insulin
[47]. Scapin (2018) has defined the full S2 binding site
[39], and Gutmann (2020) first observed the connection of insulin with discrete IR-S2
[42][43]. Studies have demonstrated that optimal IR activation requires multiple insulin molecules bound to S1 and S2
[48][49][48,49]. A similar result has also been presented in a study of the cryo-EM structure of the IR–insulin complex at 3.2 Å resolution
[40][42]. The binding of insulin to S1 of apo-IR could release the autoinhibited conformation, which was an essential step for IR activation, while binding to S2 was important for the IR to adopt the active T-shape
[50][51][52][50,51,52]. Cryo-EM analysis of the insulin–IR complex has revealed that insulin binds independently to the site of S2 between the FnIII-1 and FnIII-2 domains
[42][43]. Another study has shown that the fibronectin domain is folded inwards, in a pincer-like fashion, which brings domains FnIII-3 and FnIII-3′ into contact
[43][44].
The cryo-EM structure of the full-length human IR–insulin complex (human HEK293F cells) in the active state at an overall resolution of 3.2 Å unexpectedly revealed that a maximum of four insulin molecules can bind to the “T”-shaped IR dimer at four distinct sites
[40][42]. Furthermore, at least one insulin molecule bound to two S2s and a maximum of four insulin molecules at four sites are required to form the “T”-shaped dimer
[39][40][52][39,42,52]. Insulin 1 mainly binds to the primary site formed by the L1 domain, and α-CT then makes contact with a loop of the FnIII-1 domain from the IR promoter that donates α-CT
[40][42]. During IR activation, a tripartite interface between insulin 1 and site 1 stabilizes the active IR dimer. Insulin 2 binds to a novel binding site on the FnIII-1 domain, located on the backside of the β sheet
[40][42].
However, there is still a lack of detailed analysis of which site is connected first and how the first and second insulin binding results in different phosphorylation status of the IR
[52]. The reported findings have emphasized the importance of the conformational changes of the IR-ECD and IR–insulin complex in the insulin/insulin-like growth factor signaling (IIS) pathway. Hence, the precise mechanism of how insulin binds to the IR at first remains elusive, and further research is still needed.
2.2. Activation of the IR
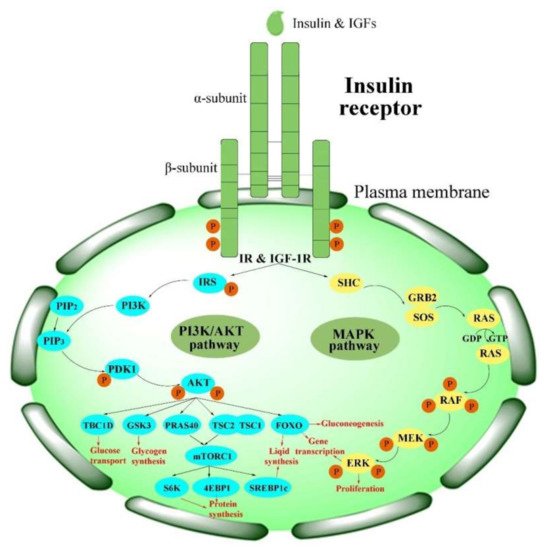
Physiologically, the function of the IR is activated in the insulin/IGF-1-like signal (IIS) pathway by the ligand
[2]. The IIS pathway is commonly known as a significant nutrient-dependent endocrine pathway and regulates numerous physiological processes, such as metabolism, growth and development, and so on
[6]. In the IIS pathway, the IR regulates two primary cell-signaling cascades (
Figure 2)
[53]: the phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway and the mitogen-activated protein kinase (MAPK) pathway (extracellular-signal regulated kinase signaling pathway (ERK))
[53][54][55][56][53,54,55,56]. The PI3K/AKT pathway is primarily responsible for controlling metabolic processes such as glucose transportation and the synthesis of lipids, proteins, and glycogen. In contrast, the MAPK pathway is primarily related to the mitogenic effects of insulin and is mainly responsible for cell growth and proliferation
[56][57][56,57].
Figure 2. Activation of the IR in insulin signaling pathways. PI3K/AKT pathways: phosphatidylinositol-3-kinase signaling pathways; MAPK pathway: mitogen-activated protein kinase pathway.
The major upstream factors of the IIS pathway are various insulin-like peptides (ILPs). Based on primary structure and receptor binding preferences, these ILPs can be subdivided into insulin, insulin-related growth factors (IGFs, including IGF-I and IGF-II) in mammals, and ILPs in insects
[58]. Insulin is a peptide hormone secreted by pancreas β-cells and is one of the most conserved molecules in animals
[59][60][59,60]. IGFs are peptides that have a homology of 40–80% with insulin. In humans, both insulin and IGFs can bind to the IR on the cell surface and functionally mediate cellular proliferation and differentiation, lipid metabolism, glucose homeostasis, and DNA synthesis
[60][61][60,61]. Meanwhile, in insects, ILPs are the most general growth-promotion signaling factors
[62][63][64][65][62,63,64,65], and evidence has suggested that ILPs are homologues of human insulin
[6][66][6,66]. Insulin is the major regulatory factor in humans, but various ILPs have been identified in different insect species, ranging from one—in the
Nevada dampwood termite,
Zootermopsis nevadensis (Hagen)—to more than 40—in the silkworm,
Bombyx mori L.
[60].
ILPs first phosphorylate the IR and then activate IR signaling. The tyrosine-phosphorylated IR, in return, recruits and phosphorylates other intracellular adaptor proteins, such as IR substrate (IRS) proteins and several other substrates, including Src homology 2 domain-containing (SHC), Grb2-associated binder (GAB), APS (SHB2), and Cbl, at several tyrosine residues
[56][67][56,67]. There are six isoforms (IRS1–6) in the IRS family
[68]; among these, IRS1 and IRS2 are the main isoforms
[68][69][68,69]. These proteins mediate the association with the Src homology 2 (SH2) domains and lead to initiation of the PI3K/AKT pathway, as well as activation of the downstream phosphoinositide-dependent kinase (PDK1) and protein kinase B (PKB, also called AKT)
[4]. Phosphorylation of the IR triggers the activation of cellular signaling pathways, which play different roles in human beings and insects.
3. Functions of the IR
3.1. The Functions of the IR in Human Beings
在人类中,I
n humans, the IR
plays a crucial role in whole-body nutrient homeostasis and in various diseases, such as 在全身营养稳态和各种疾病中起着至关重要的作用,例如AD
[8], [8],T2DM
[4][9][10], obesity [70], atherosclerosis [31], multiple cancers [11][12][13], and cardiovascular disease [71], as well as neurodegenerative disorders [14], metabolic syndrome [15] and polycystic ovary syndrome [72][4,9,10],肥胖[70],动脉粥样硬化[31],多发性癌症[11,12,13]和心血管疾病[71],以及神经退行性疾病[14],代谢综合征[15]和多囊卵巢综合征[72]].
Thus, it is necessary to understand the cellular expression and the functions of the 因此,有必要了解IR
in order to propose new treatment concepts and to develop novel drugs.的细胞表达和功能,以便提出新的治疗概念并开发新药。
The IR
mediates whole-body nutrient homeostasis and is expressed ubiquitously through the classic insulin-responsive targets in the liver, muscle, and adipose tissue [3]. A has demonstrated that the 介导全身营养稳态,并通过肝脏、肌肉和脂肪组织中经典的胰岛素反应靶标普遍表达[3]。最近的研究表明,IR
is distributed in both dendritic shafts and spines in living hippocampal brain neurons [73]. Knockout of the 分布在活海马脑神经元的树突状轴和棘中[73]。IR
resulted in many impaired target organs. Hepatic deletion of the 的敲除导致许多靶器官受损。IR
led to hyperglycemia, disorders in fatty acid metabolism, and an increase in the expression of fatty acid oxidation enzymes [74]. 的肝脏缺失导致高血糖、脂肪酸代谢紊乱以及脂肪酸氧化酶表达增加[74]。在粘膜上皮细胞中,I
n mucosal epithelial cells, the IR
interacts with the voltage-dependent anion channel-1 (VDAC1) in mitochondria. Knockdown of the IR gene triggered robust mitochondrial fragmentation and altered polarization [75], while knockout of the 与线粒体中的电压依赖性阴离子通道-1(VDAC1)相互作用。IR基因的敲除触发了强大的线粒体碎片化和极化改变[75],而β
-cell 细胞IR
gene led to impaired insulin secretion [76]. Additionally, missense mutations of the 基因的敲除导致胰岛素分泌受损[76]。此外,IR
may cause severe inherited insulin resistance syndromes [77].的错义突变可能导致严重的遗传性胰岛素抵抗综合征[77]。
The IR
is a cell-surface receptor translocating to the nucleus, and is associated strongly with RNA polymerase II in the chromatin [78]. In the cell, host cell factor是易位到细胞核的细胞表面受体,与染色质中的RNA聚合酶II密切相关[78]。在细胞中,宿主细胞因子-1
((HCF-1
) acts as a transcriptional coregulator functionally mediating the binding of the IR to specific sites located in the gene promoters [79]. )充当转录联合调节剂,功能性地介导IR与位于基因启动子中特定位点的结合[79]。HCF-1
mediates the association between the 介导IR
and DNA. HCF-1 binds to DNA indirectly through DNA sequence-specific transcription factors, and then forms a complex with the IR and 和DNA之间的关联。HCF-1通过DNA序列特异性转录因子间接结合DNA,然后在染色质中与IR和Thanatos
-associated protein domain-containing protein 11 (相关的蛋白质结构域蛋白11(THAP11
) in the chromatin. Knockdown of HCF-1 can inhibit the binding ability of the )形成复合物。HCF-1的敲低可抑制IR与启动子的结合能力[79]。另一项研究表明,妊娠期糖尿病(GDM)女性皮下和内脏脂肪组织中IR
to the pro的m
otors [79]. Another study indicated that the mRNA
and protein levels of the 和蛋白质水平明显降低[80]。IR
were obviously reduced in the subcutaneous and visceral adipose tissue of wom
en with gestational diabetes mellitus (GDMs) [80]. The decrease in IR mRNA
was accompanied by a decrease in methylation levels of the 的降低伴随着IR启动子甲基化水平的降低[80]。在下丘脑中也观察到这种现象[81]。IR
promoter [80]. This phenomenon has also been observed in the hypothalamus [81]. The methylation degree of the 启动子内IR
nuclear factor 核因子I
((IRNF-I
) binding site within the IR promoter was dramatically inversely correlated with the gene level of the IR. These findings have opened a new avenue for further studies on the functions and mechanisms of the IR. More studies focusing on demonstrating whether epigenetic modifications in the IR sequence impact IR expression of the IR are needed [82].)结合位点的甲基化程度与IR的基因水平呈显着负相关。这些发现为进一步研究IR的功能和机制开辟了新的途径。需要更多研究来证明IR序列中的表观遗传修饰是否会影响IR的IR表达[82]。
3.2. 昆虫中红外线的功能
3.2. The Functions of the IR in Insects
对人类红外的研究引起了人们对昆虫中红外功能的兴趣,以及随之而来的开发具有高效率和低毒性的新型红外靶向杀虫剂的可能性。
Studies of the 在昆虫中,已经揭示了IR
in human beings have raised interest in the functions of the 的多种功能[83]。众所周知,IR
in insects and the consequent possibility for the development of new IR-targeting insecticides with high efficiency and low toxicity.
In insects, multiple functions of the IR have been revealed [83]. The IR is well-known to be implicated—either directly or by crosstalk with other major hormones such as juvenile hormone (与胚胎后发育[84,85],营养表型可塑性和体型控制[86,87],生殖和透析[55],生殖和重生[55]以及昼夜节律和行为[88,89,90]有关,直接或通过与其他主要激素(如幼年激素(JH
) and ecdysteroids (especially )和蜕皮甾醇(尤其是20-
hydroxyecdysone, 20E)—in post-embryonic development [84][85], nutrition-based phenotypic plasticity and body size control [86][87], reproduction and diapause [55], and circadian rhythmicity and behaviors [88][89][90]羟基依蔀酮,20E)相联系[84,85],基于营养的表型可塑性和体型控制[86,87],繁殖和透析[55],以及昼夜节律和行为[88,89,90]].
The IR
is also indispensable in insect photoperiodism, lifespan, and aging due to its relation to metabolism and growth [25][91][92][93]. Overall, studies have indicated that the 在昆虫光周期、寿命和衰老中也是必不可少的,因为它与新陈代谢和生长有关[25,91,92,93]。总体而言,研究表明,IR
is indispensable in insect growth [94], development and reproduction [95][96], polymorphism [24], lifespan [97], and oviposition [98]. Therefore, the 在昆虫生长[94],发育和繁殖[95,96],多态性[24],寿命[97]和产卵[98]中是不可或缺的。因此,IR
represents an important target for the management of pests and parasites.
是害虫和寄生虫管理的重要目标。