Microalgal biomass and metabolites can be used as a renewable source of nutrition, pharmaceuticals and energy to maintain or improve the quality of human life. Microalgae’s high volumetric productivity and low impact on the environment make them a promising raw material in terms of both ecology and economics. To optimize biotechnological processes with microalgae, improving the productivity and robustness of the cell factories is a major step towards economically viable bioprocesses. The success of a random mutagenesis approach using microalgae is determined by multiple factors involving the treatment of the cells before, during and after the mutagenesis procedure. Using photosynthetic microalgae, the supply of light quality and quantity, as well as the supply of carbon and nitrogen, are the most important factors. Besides the environmental conditions, the type of mutagen, its concentration and exposure time are among the main factors affecting the mutation result.
- random mutagenesis
- algae
- mutagens
- strain development
- microalgal biotechnology
1. Physical Mutagens in Microalgal Biotechnology
1.1. Ultraviolet Light
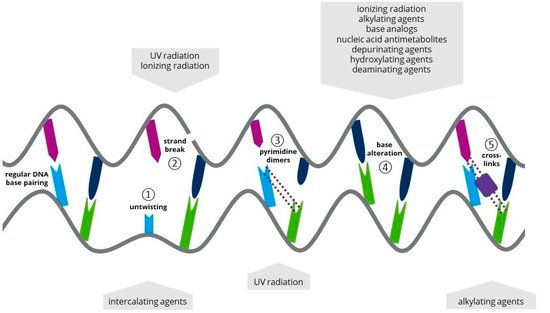
1.2. Ionizing Radiation
1.3. Atmospheric and Room Temperature Plasma
1.4. Laser Radiation
| Mutagen | Method, Exposure Time, Source, Distance, Recovery Time | Reference Microalgae | Mutation Results | References | ||
|---|---|---|---|---|---|---|
| Mutated trait |
WT * | M ** | ||||
| UV | UV 18 W, for 13 min, 15 cm, 24 h darkness | Chlorella vulgaris Y-019 |
neutral lipid accumulation | |||
2.5. Other Approaches for Chemical Mutagenesis
Table 2. Chemical mutagens applied on microalgae.
| Mutagen | Method, Exposure Time, Source, Distance, Recovery Time | Mutagen Concentration, Time of Exposure | Reference Microalgae | Mutation Results | References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutated trait |
WT * | M ** | ||||||||||||||||||
| EMS | EMS 0.1–1.2 Mfor 60 min | Nannochloropsis sp. | fatty acid methyl esters g/g of dry wt] | Mutated trait |
0.123 | WT * | 0.238 | M ** | [101] | |||||||||||
| [g/g dry wt] | 0.11 | 0.26 | [ | 36 | ] | |||||||||||||||
| EMS 0.4–1 g/Lfor 60–120 min | EMS | Haematococcus pluvialis | EMS 0.1–1.2 M for 60 min |
total carotenoid; Astaxanthin[g/g of dry wt] | Nannochloropsis sp. | 0.02; 0.005 | fatty acid methyl esters [g/g of dry wt] | 0.02;0.019 | 0.123 | [102] | 0.238 | [101] | UV-C | UV-C 253.7 nm, 30-W, 3–30 min, 9 cm, 24 h darkness | Chlorella sp. | protein content [g/L] | ||||
| EMS 300 mM for 60 min | 0.0242 | EMS 0.4–1 g/L for 60–120 min | 0.0688 | Chlorella vulgaris | Haematococcus pluvialis | protein content [g/g of dry wt] | total carotenoid; Astaxanthin [g/g of dry wt][37 |
0.353 | 0.02; 0.005] | |||||||||||
| 0.455 | 0.02; | 0.019 | [ | 34] | [102] | UV-C 254 nm 1.4 mW/cm2 for 60 s, 15 cm, 16 h darkness | Chlorella vulgaris | fatty acids 16:0;18:0, 20:0 [% of total fatty acids] | 27.9; 3.9; 11.9 | |||||||||||
| EMS 0.2–0.4 M for 2 h in darkness | EMS 300 mM for 60 min | Chlorella vulgaris | violaxanthin [mg/L culture] | 47.4; 5.9; 19.9 | protein content [g/g of dry wt][68] | |||||||||||||||
| 1.64 | 0.353 | 5.23 | 0.455 | [ | 103] | [34] | UV-C 254 nm, 15 W, (Vilber–Lourmat, France), for 30–180 s, 5 cm, 24 h darkness | natural isolates of photosynthetic microorganism | lipid content though Nile red autofluorescence; with fluorescence emission | 35; 1081 | ||||||||||
| EMS 0.1–0.2 M | EMS 0.2–0.4 M for 2 h in darkness | 983; 89,770 | Phaeodactylum tricornutum | Chlorella vulgaris | [38 | total carotenoids [g/g dry wt] | violaxanthin [mg/L culture]] | |||||||||||||
| 0.009 | 1.64 | 0.011 | 5.23 | [ | 104] | [103] | UV-C 40,000 μJ/cm, 254 nm, overnight darkness | Scenedesmus obliquus | trans-fatty acid productivity [g/(L·d)] |
0.095 | 0.112 | [69] | ||||||||
| EMS 0.2 M for2 h in the dark | EMS 0.1–0.2 M | Dunaliella tertiolecta | Phaeodactylum tricornutum | Zeaxanthin [μg/106·cells] | total carotenoids [g/g dry wt] | 0.131 | 0.009 | 0.359 | 0.011 | [105] | [104] | UV-C 254 nm 340 mW cm2, for 3–32 min, 13.5 cm, 24 h darkness |
Isochrysis affinis galbana | total fatty acid [g/g dry wt] |
0.262 | 0.409 | [40] | |||
| EMS 20–40 µL/mL for 2 h | EMS 0.2 M for 2 h in the dark |
Chlamydomonas | Dunaliella tertiolecta | reinhardtii | fatty acid methyl esters yield [%] | Zeaxanthin [μg/106·cells] | 6.53 | 0.131 | 7.56 | 0.359 | [106] | [105] | UV-C, for 1–10 min, 40 cm, overnight darkness | Chlorella vulgaris | ||||||
| EMS 0.2 M for2 h in the dark | lipid content [g/g] | EMS 20–40 µL/mL for 2 h | Dunaliella | 0.58 | Chlamydomonas | salina | 0.75 |
reinhardtii | carotenoid synthesis [Mol Car/Mol Chl] | fatty acid methyl esters yield [%] | 0.99 | 6.53[35] | ||||||||
| 1.24 | 7.56 | [ | 107 | ] | [106] | Gamma irradiation | ||||||||||||||
| EMS 100 μ mol mL−1, for 30 min | EMS 0.2 M for 2 h in the dark10 doses of irradiation 50–7000 kGy, 60Co gamma ray irradiator, room temperature |
Scenedesmus sp. | Chlorella sp | lipid productivity [g/L·d] |
0.0648 | Dunaliella0.097 | . | salina |
lipid content (g/g of dry wt]; productivity [g/(L·d)] | carotenoid synthesis [Mol Car/Mol Chl] | 0.247; 0.1536 | 0.99[ | 0.356; 0.2487 | 1.24 | [108] | [107]70] | ||||
| ARTP | He RF power 100 W, plasma temperature 25–35 °C, for 20; 40; 60 and 80 s, 2 mm | |||||||||||||||||||
| EMS 100 μ mol mL−1, for 30 min | EMS 0.4M, for 60 min | Chlorella sp.Spirulina platensis | Carbohydrates productivity [g/L·d] |
0.0157 | Coelastrum | 0.026 | sp. | lipid content [g/g of dry wt]; productivity [g/(L·d)] | Astaxanthin content [g/L] | 0.247; 0.1536 | 0.0145 | 0.356; 0.2487 | 0.0283 | [108[59] | ||||||
| ] | [ | 109 | ] | He RF power 100 W, plasma temperature 25–35 °C, 20–60 s, 2 mm | Chlamydomonas reinhardtii | H2 production [mL/L] | ~16.1 | |||||||||||||
| EMS + UV | 84.1 | EMS 0.4 M, for 60 min | [ | 71 | ] | |||||||||||||||
| UV + EMS 25 mM for 60 min | Coelastrum sp. | Chlorella vulgaris | Astaxanthin content [g/L] | lipid content [%] | 0.0145 | 100 | 0.0283 | 167 | [109] | [85] | He RF power 150 W, for 100 s | Crypthecodinium cohnii | biomass concentration [g dry wt/L] |
3.60 | 4.24 | [72] | ||||
| UV 5–240 s, 245 nm + EMS 0.24 mol/L for 30 min | EMS + UV | Nannochloropsis salina | UV + EMS 25 mM for 60 min | fatty acid methyl ester [g/g of dry wt] | Chlorella vulgaris | 0.175 | lipid content [%] | 0.787 | 100 | [110] | 167 | [85] | Heavy ion beam | 12 C6+ ion beam 31 keVµm−1 160 Gy, | Nannochloropsis oceanica | |||||
| MNNG | lipid productivity [g/L·d] | UV 5–240 s, 245 nm + EMS 0.24 mol/L for 30 min | 0.211 | 0.295 | MNNG 0.1 mM for 60 min | Nannochloropsis salina | Haematococcus pluvialis | fatty acid methyl ester [g/g of dry wt] | Total carotenoid content [g/L] | 0.175 | ~0.067 | 0.787[73] | ||||||||
| 0.089 | [ | 110 | ] | [ | 80 | ] | 12 C6+ ion beam, 90 Gy | Desmodesmus sp. | lipid productivity [g/L·d] | 0.247 | ||||||||||
| MNNG 5 µg/mL for 60 min | MNNG | Chlorella sp. | MNNG 0.1 mM for 60 min | max. growth rate under alkaline conditions [ d−1] | Haematococcus pluvialis0.298 | 0.064 | Total carotenoid content [g/L][74] | |||||||||||||
| 0.554 | ~0.067 | [ | 111 | ] | 0.089 | [80] | Low-energy ion beam implementation | N+ ion beam chamber pressure 10−2 Pa Dose of implantation 0.3–3.3·1015 ions cm−2 s−1 |
Chlorella pyrenoidosa | lipid productivity [g/ L·d]; Lipid content [g/g dry wt] | 47.7; 0.337 | 64.4; 0.446 | [75] | |||||||
| MNNG 0.02 mol/Lfor 60 min | MNNG 5 µg/mL for 60 min | Nannochloropsis | Chlorella | oceanica | sp. | Total lipidcontent [g/g]Lipid productivity [g/(L·d)] | max. growth rate under alkaline conditions [ d−1] | 0.241; 0.0065 | 0.064 | 0.299; 0.0086 | 0.554 | [33] | [111] | laser radiation | He–Ne laser 808 nm, 6 W, 4 min, 24 h darkness | C. pyrenoidesa | lipid content [g/g dry wt] | 0.354 | 0.780 | [66] |
| MNNG 0.1–0.2 M | MNNG 0.02 mol/L for 60 min |
Phaeodactylum tricornutum | Nannochloropsis oceanica |
total carotenoids [g/g dry wt] | Total lipid content [g/g] Lipid productivity [g/(L·d)] |
0.009 | 0.241; 0.0065 | 0.011 | 0.299; 0.0086 | [104] | [33] | Nd:YAG laser 1064 nm, 40 mW 8 min, 24 h darkness | Chlorella vulgaris | lipid content [g/g dry wt] | 0.315 | 0.525 | [66] | |||
| Nd:YAG laser 1064 nm, 40 mW 2 min, 24 h darkness | Chlorella pacifica | lipid content [g/ L]−1] |
0.033 | 0.088 | [76] | |||||||||||||||
| semiconductor laser 632 nm, 40 mW, 4 min, 24 h darkness |
Chlorella pacifica | lipid content [g/ L]−1] |
0.033 | 0.077 | [76] | |||||||||||||||
2. Chemical Mutagens in Microalgal Biotechnology
2.1. Alkylating Agents as a Chemical Mutagen
2.2. Base Analogs (BAs) as a Chemical Mutagen
2.3. Antimetabolites (AMs) as a Chemical Mutagen
2.4. Intercalating Agents (IAs) as a Chemical Mutagen
| MNNG 0.2 mg/mL | |||||||||||||
| MNNG 0.1–0.2 M | |||||||||||||
| Chlorella sorokiniana | |||||||||||||
| Phaeodactylum tricornutum | |||||||||||||
| Lutein content [g/L] | |||||||||||||
| total carotenoids [g/g dry wt] | |||||||||||||
| 0.025 | |||||||||||||
| 0.009 | |||||||||||||
| 0.042 | |||||||||||||
| 0.011 | |||||||||||||
| [ | |||||||||||||
| 83 | ] | [ | 104 | ] | |||||||||
| MNNG 0.2 mg/mL | MNNG 0.25–0.5 mM | Chlorella sorokiniana | Botryosphaerella sp. | Lutein content [g/L] | lipid[g dry wt/(m2 day)]; biomass productivity [g dry wt/(m2·day)] | 0.025 | 1.0; 3.2 | 0.042 | 1.9; 5.4 | [83] | [84] | ||
| NMU | MNNG 0.25–0.5 mM | NMU 5 mM for60–90 min | Botryosphaerella sp. | Nannochloropsis oculata | lipid [g dry wt/(m2 day)]; biomass productivity [g dry wt/(m2·day)] | Total fatty acid [g/g dry wt] | 1.0; 3.2 | 0.0634 | 1.9; 5.4 | 0.0762 | [84] | [82] | |
| DES + UV | NMU | UV 7–11 min 254 nm +DES 0.1–1.5% (V/V) 40 min | NMU 5 mM for 60–90 min |
Haematococcus pluvialis | Nannochloropsis oculata | astaxanthin content [mg/L] | Total fatty acid [g/g dry wt] | ~0.031 | 0.0634 | ~0.089 | 0.0762 | [81] | [82] |
| 5BU | DES + UV | 5BU 1 mM for 48 h | UV 7–11 min 254 nm + DES 0.1–1.5% (V/V) 40 min |
Chlamydomonas reinhardtii | Haematococcus pluvialis | O2 tolerance [%] | astaxanthin content [mg/L] | 100 | ~0.031 | 1400 | ~0.089 | [112] | [81] |
| 5′FDU | 5BU | 5′FDU 0.25 and 0.50 mM for 1 week | 5BU 1 mM for 48 h | Chlorella vulgaris | Chlamydomonas reinhardtii | fatty acids 16:0; 18:0; 20:0 [% of total fatty acids] | O2 tolerance [%] | 27.9; 3.9; 11.9 | 100 | 46.9; 5.5; 18.5 | 1400 | [68] | [112] |
| Acriflavin | 5′FDU | Acriflavin 2–8 μg/mL for 1–3 d in darkness | 5′FDU 0.25 and 0.50 mM for 1 week | Chlamydomonas reinhardtii zyklo | Chlorella vulgaris | Loss of respiratory rate [nmol O2/(min·107 cells)] through loss of mitochondrial DNA | fatty acids 16:0; 18:0; 20:0 [% of total fatty acids] |
23.2 | 27.9; 3.9; 11.9 | 3.7 | 46.9; 5.5; 18.5 | [100] | [68] |
| Acriflavin | Acriflavin 2–8 μg/mL for 1–3 d in darkness | Chlamydomonas reinhardtii zyklo | Loss of respiratory rate [nmol O2/(min·107 cells)] through loss of mitochondrial DNA | 23.2 | 3.7 | [100] |
3. Further Approaches in Random Mutagenesis
Recently, combined mutagenesis approaches have generated high interest as results indicated that they have a higher success rate than individual approaches. For instance, Wang et al. [81] applied a two-step random mutagenesis protocol to Haematococcus pluvialis cells using first UV irradiation, then EMS and DES mutagenesis, causing astaxanthin production to increase by a factor of 1.7 compared to the wild strain. Beacham et al. [110] used a reverse protocol for Nannochloropsis salina, starting with exposure to EMS, followed by UV irradiation, yielding a three-fold increase in cellular lipid accumulation. Comparable results were achieved by Sivaramakrishnan and Incharoensakdi [113], who exposed Scenedesmus sp. to UV irradiation in combination with oxidative stress by H2O2.
Other approaches can be used to select desired microalgal cells if the results obtained by random mutagenesis are insufficient. Among them, Adaptive Laboratory Evolution (ALE) is commonly used to adapt the physiology of cells to specific process conditions, such as high temperatures [114]. Its principle is based on natural selection, as presented in the Darwinian Theory, on the laboratory bench [115], and includes extensive cultivation in a specifically designed lab environment so that enhanced phenotypes can be selected after a long period of time [116]. The environmental conditions that can be altered include light irradiation, lack of nutrients, such as nitrogen, osmotic, temperature and oxidative stress [115,117,118]. Connecting the results of ALE with whole genome sequencing and “omics” methods enables gene functions to be discovered easily [116]. However, ALE does not prevent gene instability that might occur more often than in randomly mutated cells [114,117].
Additional environmental factors can be applied on microalgae; for example, Miazek et al. [119] reviewed the use of metals, metalloids and metallic nanoparticles to enhance cell characteristics. Moreover, phytohormones or chemicals acting as metabolic precursors have already been applied to microalgae [120]. A discussion of the methods used in the latter case exceeds the scope of this review.
More recently, a new technique was developed, known as Space Mutation Breeding (SMB). This technique may have direct or indirect effects on the growth and metabolic activities of microalgae, due to the unusual environment of space, characterized by high-energy ionic radiation, space’s magnetic field, ultra-high vacuum and microgravity [121]. The SMB technique provides some advantages, such as the great improvement in species' qualities in a short time [122]. This was achieved by Chen Zishuo et al. [121], with a seawater Arthrospira platensis mutant, yielding a sugar content 62.26% higher than the wild type.
Reference
