The illegal use of β-adrenergic agonists during livestock growth poses a threat to public health; the long-term intake of this medication can cause serious physiological side effects and even death. Therefore, rapid detection methods for β-adrenergic agonist residues on-site are required. Traditional detection methods such as liquid chromatography have limitations in terms of expensive instruments and complex operations. In contrast, paper methods are low cost, ubiquitous, and portable, which has led to them becoming the preferred detection method in recent years. Various paper-based fluidic devices have been developed to detect β-adrenergic agonist residues, including lateral flow immunoassays (LFAs) and microfluidic paper-based analytical devices (μPADs).
- β-adrenergic agonists
- paper-based device
- lateral flow immunoassay
- point-of-care testing
- biosensors
- microfluidics
1. Introduction

2. LFA Formats and Principles
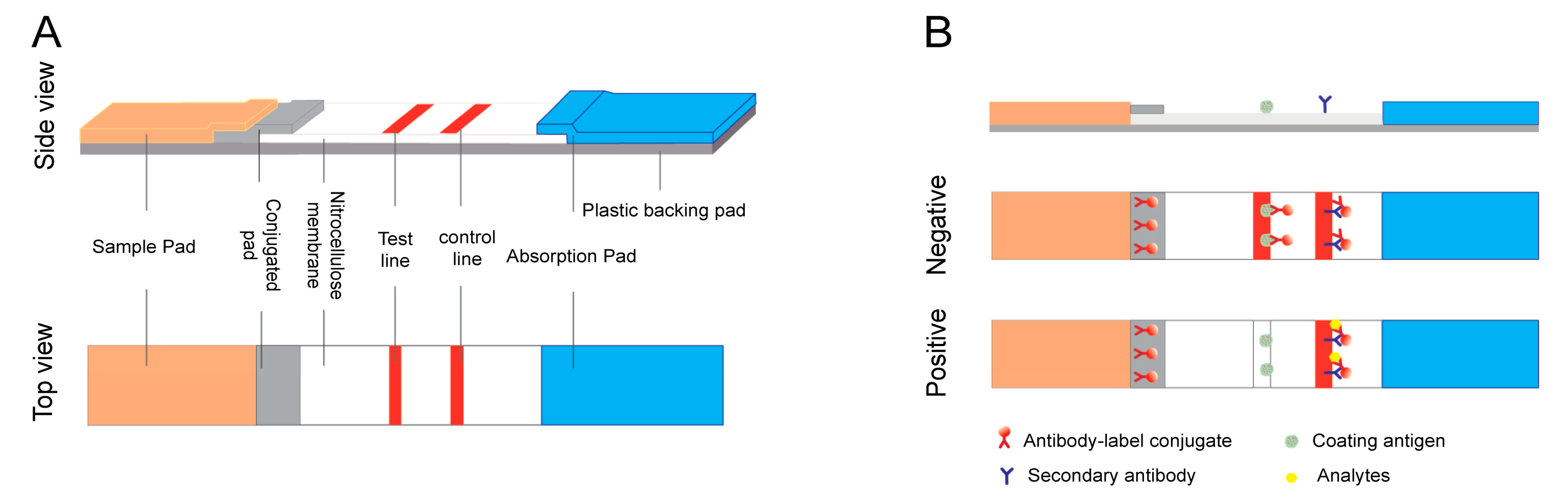
2.1. Sandwich Format
2.2. Competitive Format
3. Applications for Detecting β-Adrenergic Agonist Residues
β-adrenergic agonists are a group of synthetic phenethanolamine compounds, and according to their different aromatic ring structures they can be separated into three categories: aniline, phenol, and resorcinol [24]. LFA technology has been adequately developed to detect β-adrenergic agonists. As they are low molecular mass compounds, competitive assays should be used, the principles of which were described in Section 2 Section 2 of this entreviewy (2. LFA formats and principles). If the analyte is not present in the sample, then the labeled antibody will be caught by the bovine serum albumin (BSA)-analyte and immobilized on the membrane to form a clear test line with negative results; if the analyte is present in the sample exceeding the lower detectable concentration, it competes with the BSA-analyte immobilized on the test line and binds to the finite amount of labeled antibody, leading to an invisible test line with positive results. Published reports on LFA applications in this field are summarized in Table 1.| Analyte | Label | Assay Format | Sample | Assay Time | LOD | Reference |
|---|---|---|---|---|---|---|
| CLE 1 and RAC 3 | AuNPs 2 | Competitive LFA | Swine urine | 5 min | 0.1 ± 0.01 ng/mL | [25] |
| CLE | AuNPs | Competitive LFA | Swine livers | 10 min | NA | [26] |
| CLE | AuNPs | Competitive LFA | Swine urine | 10 min | 3 ng/mL | [27] |
| CLE | AuNPs | Competitive LFA | Swine urine | 10 min | 0.1 ng/mL | [28] |
| CLE, RAC, SAL 4 | AuNPs | Competitive LFA | Swine urine | 10 min | 0.5 ng/mL | [29] |
| CLE | AuNPs | Competitive LFA | Swine urine | 10 min | 220 pg/mL | [30] |
| SAL | AuNPs | Competitive LFA | Swine urine | 10 min | 80 ng/mL | [31] |
| RAC | AuNPs | Competitive LFA | Swine urine | 5 min | 0.1 ng/mL | [32] |
| SAL | AuNPs | Competitive LFA | Meat and milk | 10 min | meat: 4.0 ng/g milk: 3.0 ng/g |
[33] |
| RAC | AuNPs | Competitive LFA | Swine urine | 45 min | 0.13 ng/mL | [34] |
| CLE | SeNPs 5 | Competitive LFA | Swine urine | / | 3 ng/mL | [35] |
| RAC and SAL | SeNPs | Competitive LFA | Swine urine | 5 min | RAC: 1 ng/mL SAL: 3 ng/mL |
[36] |
| CLE and RAC | SiNPs 6 | Competitive LFA | / | 10 min | CLE: 3 ng/mL RAC: 2 ng/mL |
[37] |
| CLE | SiNPs | Competitive LFA | PBS, urine, and pork | 10 min | PBS: 3 ng/mL urine: 6 ng/mL pork: 5 ng/mL |
[38] |
| Zilpaterol | AuNPs | Competitive LFA | Feed | 10 min | 20 ng/g | [39] |
| PA 7 | AuNPs | Competitive LFA | Swine urine | 10 min | 0.188 ng/mL | [40] |
| Clorprenaline | AuNPs | Competitive LFA | Swine urine | 3–5 min | 0.104 ng/mL | [41] |
| Clorprenaline | AuNPs | Competitive LFA | Swine urine | 9 min | 0.15 ng/mL | [42] |
References
- Evans, T.; Chapple, N. The animal health market. Nat. Rev. Drug Discov. 2002, 1, 937–938.
- Cabello, F.C.; Godfrey, H.P. Even therapeutic antimicrobial use in animal husbandry may generate environmental hazards to human health. Environ. Microbiol. 2016, 18, 311–313.
- Mersmann, H.J. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 1998, 76, 160–172.
- Lv, R.; Wu, E.; Wu, R.; Shen, W.; Ma, C.; Shi, R.; Guo, R.; Shao, M.; Liu, J. Sensitive detection of clenbuterol by hybrid iridium/silicon nanowire-enhanced laser desorption/ionization mass spectrometry. J. Mater. Chem. B 2020, 8, 7792–7800.
- Barbosa, J.; Cruz, C.; Martins, J.; Silva, J.M.; Neves, C.; Alves, C.; Ramos, F.; Noronha Da Silveira, M.I. MIN: Food poisoning by clenbuterol in Portugal. Food Addit. Contam. 2005, 22, 563–566.
- Kuiper, H.A.; Noordam, M.Y.; van Dooren-Flipsen, M.M.; Schilt, R.; Roos, A.H. Illegal use of beta-adrenergic agonists: European Community. J. Anim. Sci. 1998, 76, 195–207.
- Song, C.; Zhi, A.; Liu, Q.; Yang, J.; Jia, G.; Shervin, J.; Tang, L.; Hu, X.; Deng, R.; Xu, C.; et al. Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip. Biosens. Bioelectron. 2013, 50, 62–65.
- Bulletins, M. The Catalogue of Drug Banned to Use in the Feed and Animal Drinking Water. 2002. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-03/China-176.pdf (accessed on 17 June 2022).
- Zhang, X.; Zhao, H.; Xue, Y.; Wu, Z.; Zhang, Y.; He, Y.; Li, X.; Yuan, Z. Colorimetric sensing of clenbuterol using gold nanoparticles in the presence of melamine. Biosens. Bioelectron. 2012, 34, 112–117.
- Wang, W.; Zhang, Y.; Wang, J.; Shi, X.; Ye, J. Determination of beta-agonists in pig feed, pig urine and pig liver using capillary electrophoresis with electrochemical detection. Meat Sci. 2010, 85, 302–305.
- Hu, Y.; Liu, R.; Li, Y.; Li, G. Investigation of ractopamine-imprinted polymer for dispersive solid-phase extraction of trace beta-agonists in pig tissues. J. Sep. Sci. 2010, 33, 2017–2025.
- Wang, P.-L.; Fan, L.; Su, X.-O.; Ye, Z.-H. Determination of four kinds of β-agonists in swine urine by molecularly imprinted solid phase extraction followed gas chromatography coupled mass spectrometry. Chin. J. Anal. Chem. 2012, 40, 470–473.
- Shao, B.; Jia, X.; Zhang, J.; Meng, J.; Wu, Y.; Duan, H.; Tu, X. Multi-residual analysis of 16 β-agonists in pig liver, kidney and muscle by ultra performance liquid chromatography tandem mass spectrometry. Food Chem. 2009, 114, 1115–1121.
- Bocca, B.; Fiori, M.; Cartoni, C.; Brambilla, G. Simultaneous determination of Zilpaterol and other beta agonists in calf eye by gas chromatography/tandem mass spectrometry. JAOAC Int. 2003, 86, 8–14.
- McPartlin, D.A.; O’Kennedy, R.J. Point-of-care diagnostics, a major opportunity for change in traditional diagnostic approaches: Potential and limitations. Expert Rev. Mol. Diagn. 2014, 14, 979–998.
- Zhu, X.; Sarwar, M.; Yue, Q.; Chen, C.; Li, C.-Z. Biosensing of DNA oxidative damage: A model of using glucose meter for non-glucose biomarker detection. Int. J. Nanomed. 2017, 12, 979–987.
- Wang, X.-L.; Wang, L.; Hasi, C.-L.; Wang, Y.-P.; Khan, A.; Ren, B.-Z.; Liu, Z.-Z.; Hou, S.-L.; Yang, L.-H.; Zhang, L.-Y.; et al. A rapid colloidal gold immunochromatographic assay for the diagnosis of coronavirus disease 2019. Chin. Med. J. (Engl.) 2020, 133, 1986–1988.
- Lin, L.-K.; Uzunoglu, A.; Stanciu, L.A. Aminolated and Thiolated PEG-Covered Gold Nanoparticles with High Stability and Antiaggregation for Lateral Flow Detection of Bisphenol A. Small 2018, 14, 1702828.
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. Engl. 2007, 46, 1318–1320.
- Clarke, O.; Goodall, B.; Hui, H.; Vats, N.; Brosseau, C. Development of a SERS-based rapid vertical flow assay for point-of-care diagnostics. Anal. Chem. 2017, 89, 1405–1410.
- Raeisossadati, M.J.; Danesh, N.M.; Borna, F.; Gholamzad, M.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Lateral flow based immunobiosensors for detection of food contaminants. Biosens. Bioelectron. 2016, 86, 235–246.
- Ngom, B.; Guo, Y.; Wang, X.; Bi, D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: A review. Anal. Bioanal. Chem. 2010, 397, 1113–1135.
- Chen, W.; Huang, Z.; Hu, S.; Peng, J.; Liu, D.; Xiong, Y.; Xu, H.; Wei, H.; Lai, W. Invited review: Advancements in lateral flow immunoassays for screening hazardous substances in milk and milk powder. J. Dairy Sci. 2019, 102, 1887–1900.
- Wang, R.; Zhang, W.; Wang, P.; Su, X. A paper-based competitive lateral flow immunoassay for multi β-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Mikrochim. Acta 2018, 185, 191.
- Zhang, M.-Z.; Wang, M.-Z.; Chen, Z.-L.; Fang, J.-H.; Fang, M.-M.; Liu, J.; Yu, X.-P. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Anal. Bioanal. Chem. 2009, 395, 2591–2599.
- Lai, W.H.; Fung, D.Y.; Xu, Y.; Xiong, Y.H. Screening procedures for clenbuterol residue determination in raw swine livers using lateral-flow assay and enzyme-linked immunosorbent assay. J. Food Prot. 2008, 71, 865–869.
- Lai, W.; Xu, Y.; Fung, D.Y.; Xiong, Y. Development of a lateral-flow assay for rapid screening of the performance-enhancing sympathomimetic drug clenbuterol used in animal production; food safety assessments. Asia Pac. J. Clin. Nutr. 2007, 16, 106–110.
- Zhang, G.P.; Wang, X.N.; Yang, J.F.; Yang, Y.Y.; Xing, G.X.; Li, Q.M.; Zhao, D.; Chai, S.J.; Guo, J.Q. Development of an immunochromatographic lateral flow test strip for detection of beta-adrenergic agonist Clenbuterol residues. J. Immunol. Methods 2006, 312, 27–33.
- Wu, Q.; Song, Q.; Wang, X.; Yao, L.; Xu, J.; Lu, J.; Liu, G.; Chen, W. Simultaneous Detection of Multiple β-Adrenergic Agonists with 2-Directional Lateral Flow Strip Platform. Anal. Sci. 2020, 36, 653–658.
- Li, C.; Luo, W.; Xu, H.; Zhang, Q.; Xu, H.; Aguilar, Z.P.; Lai, W.; Wei, H.; Xiong, Y. Development of an immunochromatographic assay for rapid and quantitative detection of clenbuterol in swine urine. Food Control. 2013, 34, 725–732.
- Khamta, Y.; Pattarawarapan, M.; Nangola, S.; Tayapiwatana, C. Development of immunochromatographic assay for the on-site detection of salbutamol. J. Immunoass. Immunochem. 2009, 30, 441–456.
- Liu, A.; Lin, J.; Dai, M.; Wu, Y.; Fang, J.; Zhang, M. Development of a monoclonal antibody-based immunochromatographic assay detecting ractopamine residues in swine urine. Food Analytical. Methods 2016, 9, 2016–2025.
- Zvereva, E.; Zherdev, A.; Xu, C.; Dzantiev, B. Highly sensitive immunochromatographic assay for qualitative and quantitative control of beta-agonist salbutamol and its structural analogs in foods. Food Control. 2018, 86, 50–58.
- Ren, M.L.; Chen, X.L.; Li, C.H.; Xu, B.; Liu, W.J.; Xu, H.Y.; Xiong, Y.H. Lateral flow immunoassay for quantitative detection of ractopamine in swine urine. Biomed. Environ. Sci. 2014, 27, 134–137.
- Wang, Z.; Jing, J.; Ren, Y.; Guo, Y.; Tao, N.; Zhou, Q.; Zhang, H.; Ma, Y.; Wang, Y. Preparation and application of selenium nanoparticles in a lateral flow immunoassay for clenbuterol detection. Mater. Lett. 2019, 234, 212–215.
- Wang, Z.; Zhou, Q.; Guo, Y.; Hu, H.; Zheng, Z.; Li, S.; Wang, Y.; Ma, Y. Rapid Detection of Ractopamine and Salbutamol in Swine Urine by Immunochromatography Based on Selenium Nanoparticles. Int. J. Nanomed. 2021, 16, 2059–2070.
- Chen, Y.; Huang, Z.; Hu, S.; Zhang, G.; Peng, J.; Xia, J.; Lai, W. Integrated immunochromatographic assay for qualitative and quantitative detection of clenbuterol. Anal. Biochem. 2019, 577, 45–51.
- Zhu, C.; Zhao, G.; Dou, W. Immunochromatographic assay using brightly colored silica nanoparticles as visible label for point-of-care detection of clenbuterol. Sens. Actuators B Chemical. 2018, 266, 392–399.
- Shelver, W.L.; Smith, D.J. Development of an immunochromatographic assay for the β-adrenergic agonist feed additive zilpaterol. Food Addit. Contam. Part A 2018, 35, 1519–1529.
- Dai, M.; Gong, Y.; Liu, A.; Zhang, L.; Lin, J.; Zhang, M.; Yu, X. Development of a colloidal gold-based lateral-flow immunoassay for the rapid detection of phenylethanolamine A in swine urine. Anal. Methods 2015, 7, 4130–4137.
- Jiang, W.; Zeng, L.; Liu, L.; Song, S.; Kuang, H. Development of an immunochromatographic assay for rapid detection of clorprenaline in pig urine. Food Agric. Immunol. 2018, 29, 536–547.
- Peng, T.; Zhang, F.S.; Yang, W.C.; Li, D.X.; Chen, Y.; Xiong, Y.H.; Wei, H.; Lai, W.H. Lateral-flow assay for rapid quantitative detection of clorprenaline residue in swine urine. J. Food Prot. 2014, 77, 1824–1829.
- Wang, Z.; Zhao, J.; Xu, X.; Guo, L.; Xu, L.; Sun, M.; Hu, S.; Kuang, H.; Xu, C.; Li, A. An Overview for the Nanoparticles-Based Quantitative Lateral Flow Assay. Small Methods 2022, 6, e2101143.
- Xu, X.; Xu, X.; Sun, L.; Wu, A.; Song, S.; Kuang, H.; Xu, C. An ultrasensitive colloidal gold immunosensor to simultaneously detect 12 beta (2)-adrenergic agonists. J. Chromatogr. B 2022, 1191, 123119.
