Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Punniamoorthy Thiviya.
The single cell protein (SCP) refers to the dead, dried microbial cells or total protein extracted from the pure microbial culture of algae, bacteria, filamentous fungi, unicellular algae, and cyanobacteria cultivated on different carbon sources that are used as a protein supplement in human foods or animal feeds. Many studies reported that the wastes from various fruits such as orange, sweet orange, mango, banana, pomegranate, pineapple, grapes, watermelon, papaya, and many others are potential substrates for SCP production. These SCPs can be used as a protein supplement in human foods or animal feeds.
- SCPs
- fruit wastes
- fermentation
- bioconversion
1. Single CPell Protein Production Methods
The production of SCPs involves the growth of cells in a fermenter and includes processes such as washing to separate the unused medium, pre-concentration to a suitable level, final drying, and packaging [37][1]. After fermentation, the yeast biomass is harvested and may be subjected to downstream processing steps such as washing, cell disruption, protein extraction, and purification [35][2].
Solid, semi-solid, and submerged fermentation methods are the three techniques widely used to cultivate microorganisms for SCP production [4][3]. In solid-state fermentation, microorganisms are grown on solid substrates (rice or wheat bran, rice bran, straw, fruit, and vegetable waste) in the absence of free-flowing water. Furthermore, solid-state fermentation has been extensively studied for the production of various value-added products such as SCP, feeds, enzymes, ethanol, organic acids, biologically active secondary metabolites, B complex vitamins, pigments, and flavors, amongst others [38,39,40][4][5][6]. Semi-solid fermentation is a type of solid-state fermentation in which the free liquid content is increased to facilitate nutrient availability and control fermentation [39][5]. In submerged or liquid state fermentation, substrates containing the nutrients needed for microbial growth are always used in a liquid state. Soluble sugars, molasses, liquid media, and fruit and vegetable juices are a few common substrates used in submerged fermentation [9,41][7][8]. Though the purification of the products is easier in submerged fermentation methods, it requires huge capital investment and has high operating costs [4][3].
Furthermore, fermenters are also classified based on the mode of operation; batch fermentation, fed-batch fermentation, and continuous fermentation. Microbial culture is inoculated to a fixed volume of media in a fermenter, and the broth is removed at the process end in the batch fermenter, while feeding rates control the nutrients supply in the fed-batch fermenter. Continuous fermentation is perfect for biomass production, where the fresh medium is continuously added, and the used medium and cells are harvested simultaneously [42][9]. Fermenters are equipped with aerators to supply oxygen for the aerobic process, a stirrer for mixing the medium, a thermostat for temperature control, a pH detector, and other control devices to keep different parameters required for the constant growth [4][3].
After fermentation, the biomass is washed, dried, and mixed up with animal feed or directly used. Generally, fermentation products contain only 1–5% solids. Thus, pre-concentration is required to facilitate the dehydration process. Pre-concentration can be done in several ways, including centrifugation followed by heating, filtration, and evaporation. The final product should be in a dry powder form which facilitates subsequent handling and decreases transportation costs. From an economic standpoint, drum drying and spray drying are the cheapest methods for water removal [4,37][1][3]. The final product should be light in color, highly soluble, high in nutritional value, and free of viable cells for human feeding purposes. In addition, the breakdown of cell walls and nucleic acid reduction would increase the digestibility and palatability [4,43][3][10]. Finally, the dried biomass is packed under a vacuum or nitrogen atmosphere, and the packaging method varies with manufacturers and the product type [35][2]. The basic operation in SCP production is shown in Figure 1 and shows the basic operations of SCP production.
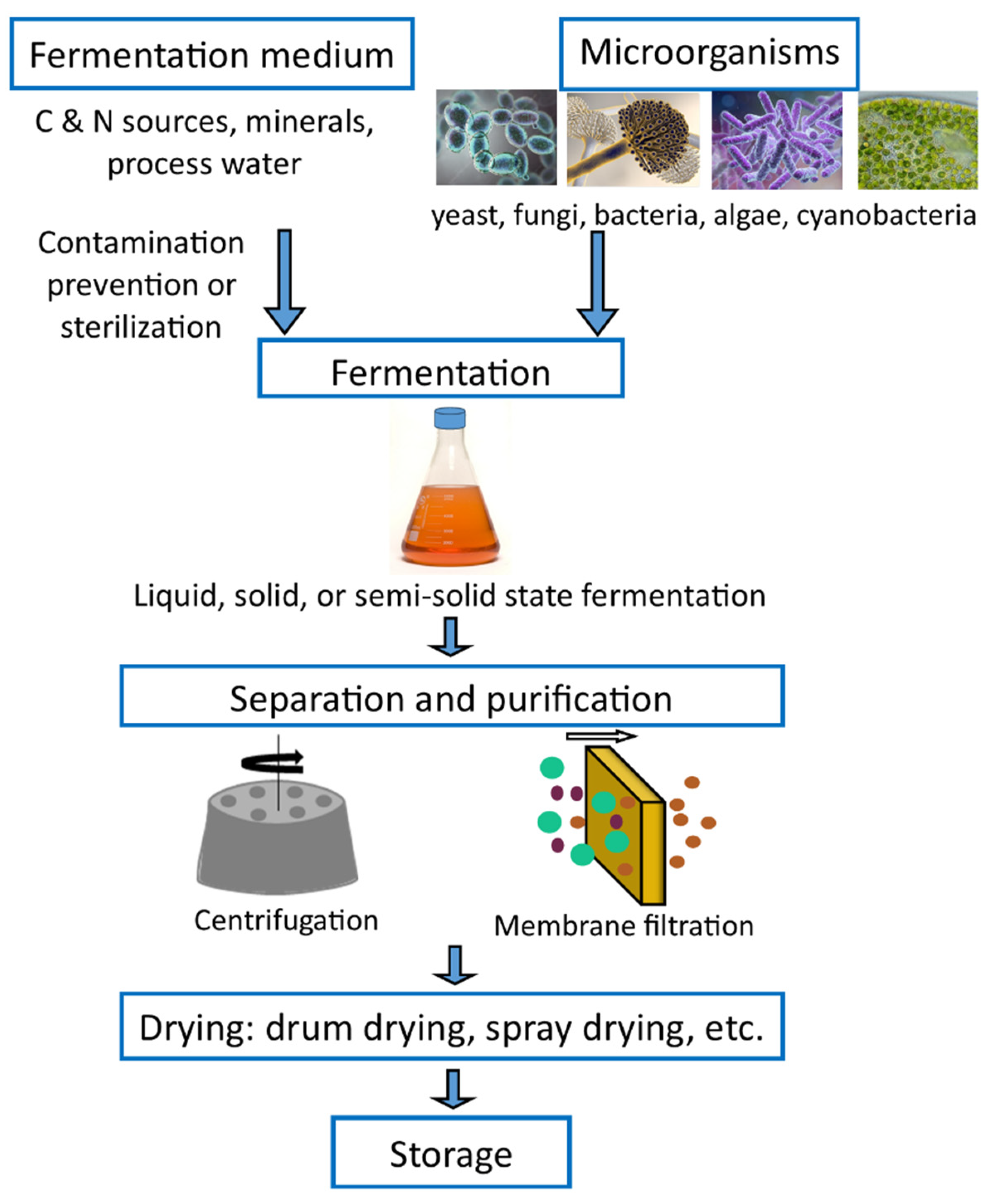
Figure 1.
Schematic diagram depicting the SCP production.
2. Fruit Production and Waste Generation
Global production of fruits has been growing steadily over the past decade, and the estimated global fruit production was 883.4 metric tons (MT) in 2019, and Asia produced 512.6 MT of fruits which contributed to 58.0% of the world production. China is the first major producer of fruits globally, followed by India, Brazil, the United States, and Mexico. In 2019, the most produced fruit in the world was bananas (116.8 MT), followed by watermelons (100.4 MT), oranges (78.7 MT), mangoes, mangosteens, and guavas (55.9 MT), pineapple (28.2 MT), citrus fruits (14.5 MT), and papaya (13.7 MT) [53][11].
A recently published WHO/FAO report recommends a minimum of 400 g of fruit and vegetables per day (excluding potatoes, cassava, and other starchy tubers) to improve health and for the prevention of non-communicable diseases including heart diseases, cancer, diabetes, and obesity, as well as for the prevention of several micronutrient deficiencies [54][12]. Increasing concern for health has led to an increase in fresh fruit consumption over the past few years [55][13]. Increasing fresh fruit consumption leads to the accumulation of fruit skins, rinds, and the residue left over at the point of consuming fruits.
Further, fruits are generally consumed directly as food or dessert. As most fruits are seasonal and have a low shelf-life, fruits are processed into various products to extend their availability all over the year. Fruits are generally processed into bottled fruits, juices, jams, marmalades, jellies, bars, pickles, canned, frozen, concentrates, dehydrated products, alcoholic beverages, and other minimally processed products [17][14].
In the recent past, intensive fruit production has caused a massive generation of fruit wastes, and the improper management of these wastes can constitute a public health risk and severe environmental problems. The main solid waste in the fruit processing industry is fruit peels [56][15]. In general, the non-edible portion of fruits and vegetables, such as peels, pods, seeds, and skins, are discarded during processing, and it accounts for about 10–60% of the total weight of the fresh produce [57][16]. Peels are the primary by-product representing almost 30% of the total weight [17][14], and can be very high in some fruits (e.g., banana 30–40%, papaya 10–20%, pineapple 29–40%, mango 25–40%, orange 30–50%) [58,59,60][17][18][19].
Traditionally fruit wastes are used as animal feed, source of fuel, fertilizers, and various other value-added novel products, including pectin, biodiesel, bioethanol, biogas, biohydrogen, bio-oil, organic acids, enzymes, polysaccharides, flavors, coloring agents, bioactive functional phytonutrients, probiotics, edible coatings, green nanoparticles, bio-degradable plastics, biochar, biosorbent, SCP, single cell oil [49,56,61,62,63,64,65][15][20][21][22][23][24][25].
The fruit processing industry generates massive waste, and the proper disposal increases processing costs. Generally, to reduce the production costs, these fruit wastes are discarded into the environment. Though the fruit wastes are biodegradable, if not processed further, these fruit wastes become spoiled rapidly and cause objectionable odor and give rise to immense environmental and health problems. Decaying fruit wastes are harbourage for microorganisms and attract pests, including flies which can cause infectious diseases and other serious health issues [13,66,67][26][27][28].
Agro-industrial wastes contain phenolic compounds and other toxic compounds, which may cause deterioration of the environment when the waste is discharged into the environment [68][29]. Fruit waste dumped in the landfills gradually rotten on landfills and releases methane, a potent greenhouse gas that traps 21 times more heat in the atmosphere than carbon dioxide [67][28]. Therefore, recycling or reusing fruit peel is a timely requirement. Using agro-wastes in SCP production can minimize environmental pollution associated with waste disposal and fulfil the world protein demand.
3. Physico-Chemical Properties of Fruit Waste
Physico-chemical composition gives an idea about the potential of fruit wastes in SCP production. The lignocellulosic fruit peel wastes contain a large number of soluble sugars, starch, fiber (cellulose, hemicelluloses, lignin, and pectin), ash, fat, protein, and other micronutrients. Liquid peel waste contains mainly simple sugars such as sucrose, glucose, and fructose and a significant amount of minerals and nitrogen content [58][17]. The solid fruit peel waste contains simple sugars (reducing and non-reducing sugars) and complex carbohydrates, such as cellulose, hemicellulose, and lignin, which can be metabolized by microorganisms [16,69][30][31]. The physico-chemical composition of fruit peel varies with fruit, types of cultivars, maturity level, geographic locations of cultivations, seasonal variations, and processing conditions (e.g., drying method, drying temperature, particle size) [17,46,70][14][32][33].
Carbohydrates are an abundant component in many fruit peels (above 50% of fruits’ dry weight) [17,71][14][34]. Dias et al., 2020 reported that pineapple contains 83% carbohydrates, while a lower value was reported with other peels such as yellow passion fruit (59%), orange (59%), and avocado peels (8%) on a dry weight basis. Dias et al., 2020 also stated that the selected fruit peels contained a significant amount of fat and ash, and the values vary with the fruit peel varieties [72][35]. Ripe banana peel contains 13.8% soluble sugar, 8% crude protein, 6.2% ether extract, and 4.8% total phenolic compounds [73][36]. Rivas et al., 2008 stated that the orange peel contains 16.9% soluble sugars, 3.8% starch, fibre (9.2% cellulose, 10.5% hemicelluloses, 42.5% pectin and 0.8% lignin),3.5% ash, 2.0% fats and 6.5% proteins in dry weight [74][37]. Orozco et al., 2014 reported that orange peel contains 14.5% hemicellulose, cellulose 11.9%, and a small amount of lignin 2.2% [75][38]. Many studies reported a low value for lignin which makes the fruit peels amenable to the hydrolysis process [74,75,76][37][38][39].
Furthermore, the use of fruit peel for the production of SCP is determined by its availability and low cost, composition, and absence of toxic substances and fermentation inhibitors [35][2]. For instance, citrus peels, such as orange peels, are rich in essential oils and limonene, a predominant component with antimicrobial property, which hinders the digestion process of microbes or fermentation process, thus resulting in less biomass production. Therefore, prior to hydrolysis, limonene is removed from the citrus bio-waste in the pre-treatment steps [20,49][20][40].
4. Fruit Waste as Substrate for Single CPell Protein Production
Fruit waste is rich in carbohydrates and other essential nutrients that could support microbial growth. Thus, fruit processing waste is a potential substrate for value-added products such as organic acids, methane/biodiesel, ethanol, enzyme, secondary metabolites, organic acids, and SCP [10,14,15][41][42][43]. SCP production has gained more attention in recent decades, and a wide variety of fruit wastes have been used as substrates. The cost and the economic viability of SCP production largely depend on substrate cost [10][41]. Hence, waste from various fruits can be a suitable substrate for SCP production. Fruit peel waste is lignocellulosic wastes [77][44] containing simple and complex sugars that can be metabolized by microorganisms [16][30]. The proximate analysis also revealed that the fruit waste contained variable amounts of carbohydrates, protein, lipid, and moisture content essential for microbial growth in SCP production [20][40].
Many studies recently aimed at producing CP from various fruit peels by using solid-state, semi-solid, and liquid-state fermentation. Fruit peels such as beles fruit, watermelon, banana, papaya, mango, sweet orange, apple, pineapple, plantain, pomegranate rind, cactus pear, and virgin grape marc are some potential substrates used for microbial growth and SCP production [18,36,78,79,80][45][46][47][48][49]. Table 1 shows the various microorganisms and fruit wastes used for SCP production.
Table 1.
SCP production using various microorganisms and fruit wastes as a substrate.
| Microorganism | Substrate (Fruit Waste) | Type of Fermentation Medium | Reference |
|---|---|---|---|
| Yeast | |||
| Yarrowia lipolytica (formerly Candida lipolytica, or Saccharomyces lipolytica) | Olive fruits wastes | SF/LSF | [81][50] |
| Candida utilis | Pineapple cannery effluent | SF/LSF | [82][51] |
| Pineapple waste | SF/LSF | [83][52] | |
| Mixture of the banana and orange waste | SF/LSF | [84][53] | |
| Orange peel | SF/LSF | [85][54] | |
| Mango wastes | SSF | [86][55] | |
| Cyberlindnera spp. | Banana peel hydrolysate | SF/LSF | [87][56] |
| Geotrichum candidum | Orange peel | SF/LSF | [88][57] |
| Saccharomyces cerevisiae | Watermelon, mixture of fruit wastes | SF/LSF | [89][58] |
| Watermelon, pineapple | SF/LSF | [90][59] | |
| Yam peel | SF/LSF | [91][60] | |
| Apple, orange peel | SF/LSF | [36][46] | |
| Cucumber peel, orange peel | SF/LSF | [20][40] | |
| Pineapple waste | SF/LSF | [11,46,92,93][32][61][62][63] | |
| Papaya waste | SF/LSF | [94][64] | |
| Apple, papaya, banana | SF/LSF | [77][44] | |
| Guava peels and cashew bagasse | SSF | [95][65] | |
| Rind of pomegranate, mango, banana, apple, sweet orange peel |
SSF | [79][48] | |
| Orange peels | SSF | [96,97][66][67] | |
| Pichia pinus | Mango waste | SF/LSF | [98][68] |
| Fungi | |||
| Aspergillus niger | Banana peel, orange peel, cucumber peel, pineapple peel, watermelon peel | SF/LSF | [29][69] |
| Banana peel | SF/LSF | [99][70] | |
| Banana peel | SF/LSF | [45][71] | |
| Banana, papaya, orange | SF/LSF | [100][72] | |
| Lemon peel, orange peel, apple pomace | SSF | [101][73] | |
| Aspergillus niger Rhizopus oryzae |
Orange peels | SSF | [97][67] |
| Aspergillus niger Saccharomyces cerevisiae |
Orange peel | SSF | [96][66] |
| Aspergillus terreus | Banana peel | SSF | [102][74] |
| Penicillium roqueforti, Penicillium camemberti | Bergamot fruit (citrus fruit) peel | SSF | [103][75] |
| Phanerochaete chrysosporium, Panus tigrinus |
Banana peel, pineapple peel, papaya peel | SF/LSF | [16][30] |
| Phanerochaete chrysosporium | Banana peels, pineapple peels, and papaya peels | SF/LSF | [16][30] |
| Rhizopus oligosporus | Papaya waste, cucumber peelings, pomegranate fruit rind, pineapple fruit skin, and watermelon skin. | SSF | [104][76] |
| Trichoderma viride, Trichoderma reesei |
Orange peel | SSF | [105][77] |
| Bacteria | |||
| Rhodococcus opacus | Orange wastes, lemon wastes | SF/LSF | [106][78] |
| Other natural sources/mixed cultures | |||
| Natural microorganisms in Palmyrah toddy | Papaya, watermelon, and banana peel | SF/LSF | [80][49] |
| Lactobacillus culture isolated from curd |
Mix fruit wastes such as pineapple peel residue, pomegranate waste, apple waste, and pear waste | SF/LSF | [107][79] |
SF/LSF, Submerged or liquid state fermentation; SSF, Solid state fermentation.
These agro-wastes used as a substrate for the selected microorganisms are composed of sugar, starch, and other cellulose materials that are metabolizable by microorganisms through the secretion of extracellular enzymes [16][30]. Lignocellulosic wastes such as agricultural residues and fruit peels are mainly composed of cellulose, hemicellulose, and lignin. Cellulose is converted into sugars, generally by the action of acids or cellulolytic enzymes. Starch materials such as wastes from corn, cassava, potatoes, and root crops are hydrolyzed to fermentable sugars by enzymes from malt or moulds. Cane, molasses, and fruit waste extract, like pineapple waste extract, contain valuable components, mainly sucrose, glucose, fructose, and other nutrients [108][80].
5. Types of Fruit Waste
5.1. Fruit Wastes Rich in Simple Sugars
SCP production depends on the type of substrate used and the composition of the culture medium. In a liquid state fermentation system, a fruit waste extract medium is used. Fruit waste extract consists of various components with a significant amount of carbohydrates, a small amount of protein, lipid, and ash [17[14][35][49],72,80], and they are rich in valuable components, mainly sucrose, glucose, fructose, and other nutrients [108][80]. Most microorganisms readily utilize simple sugars such as carbon and energy sources, and amino acids are used as nitrogen sources [109,110][81][82].
5.2. Fruit Waste Rich in Fibers
Fruit processing waste mainly consists of outer and inner shells, peels, and seeds. These fruit wastes contain fiber, and hence the waste can be categorized as structural polysaccharides-rich sources [66][27]. Large amounts of agro-industrial wastes such as bagasse, straw, stem, stalk, cobs, husk, and fruit peel are mainly composed of cellulose (35–50%), hemicellulose (25–30%), and lignin (25–30%), also being called “lignocellulosic materials” [111,112][83][84]. Typically, cellulose forms a skeleton surrounded by hemicellulose and lignin in lignocellulosic materials and acts as a protective barrier to cell destruction by bacteria and fungi (Figure 2).
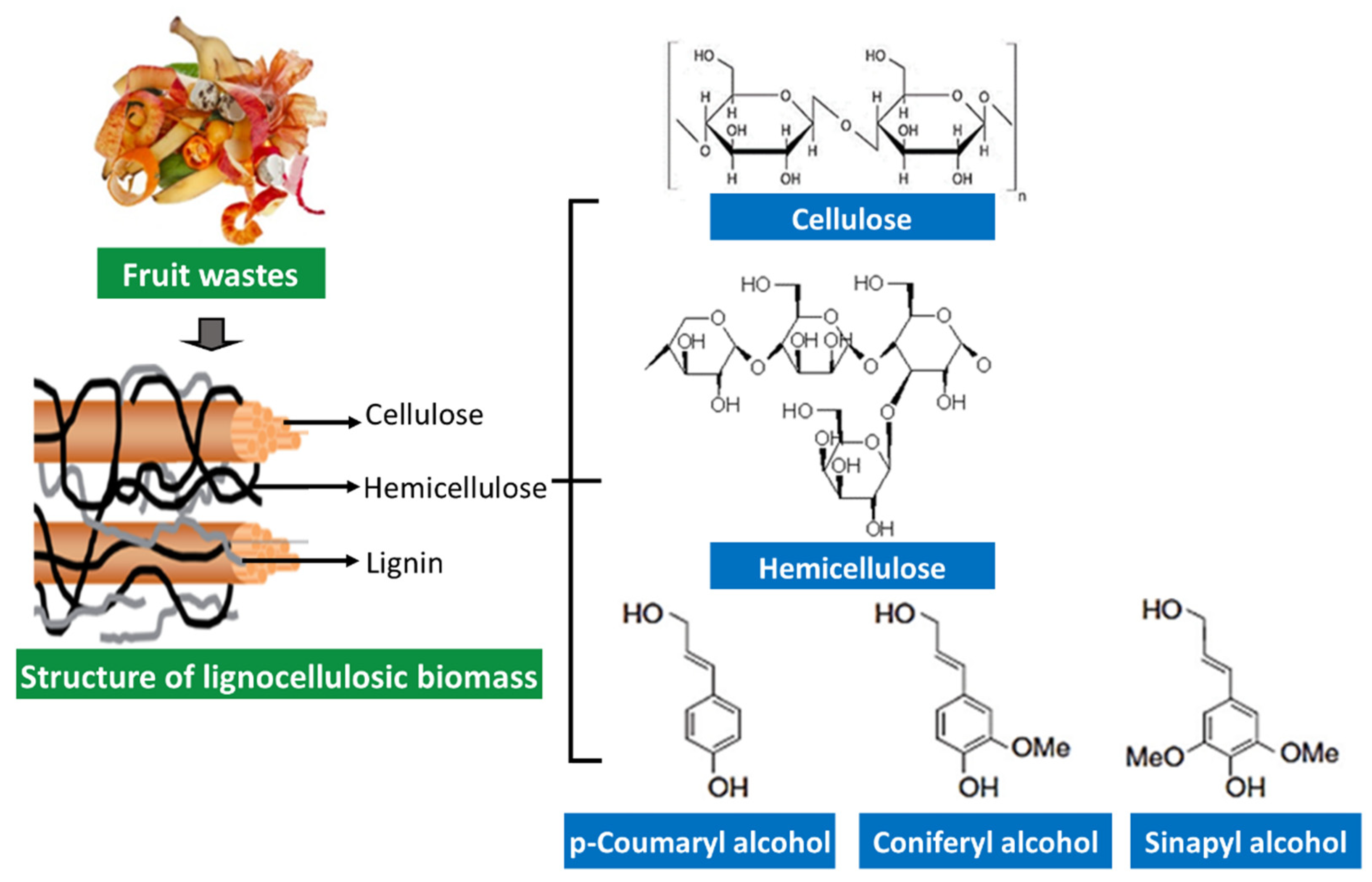
Figure 2.
Structural components of lignocellulosic biomass.
Cellulose is a homopolysaccharide composed of β-d-glucopyranose units joined via β-1,4 glycosidic linkage. The long chain cellulose polymers are linked together by hydrogen and Van der Waals bonds and packed into microfibrils [68,113][29][85]. Hemicelluloses are heterogeneous polymers that comprise five main sugars (L-arabinose, D-galactose, D-glucose, D-mannose, and D-xylose) and some organic acids (acetic and glucuronic acids). Hemicellulose has different classifications based on the main sugar in the backbone: xylans, glucans, mannans, arabinans, xyloglucans, arabinoxylans, glucuonoxylans, glucomannans, galactomannans, galactoglucomannans, and β-glucans. In contrast, lignin is not formed by sugar units but formed by a complex three-dimensional structure of phenylpropane units. Three phenyl propionic alcohols are primary monomers of lignin; p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol [68,114][29][86].
References
- Labuza, T.P.; Santos, D.B.; Roop, R.N. Engineering Factors in Single-Cell Protein Production. I. Fluid Properties and Concentration of Yeast by Evaporation. Biotechnol. Bioeng. 1970, 12, 123–134.
- Bekatorou, A.; Psarianos, C.; Koutinas, A.A. Production of Food Grade Yeasts. Food Technol. Biotechnol. 2006, 44, 407–415.
- Bajpai, P. (Ed.) Single Cell Protein Production from Lignocellulosic Biomass; Springer Briefs in Molecular Science; Springer: Singapore, 2017; ISBN 978-981-10-5873-8.
- Pandey, A. Recent Process Developments in Solid-State Fermentation. Process Biochem. 1992, 27, 109–117.
- Pandey, A.; Soccol, C.R.; Mitchell, D. New Developments in Solid State Fermentation: I-Bioprocesses and Products. Process Biochem. 2000, 35, 1153–1169.
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent Advances in Solid-State Fermentation. Biochem. Eng. J. 2009, 44, 13–18.
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. App. Sci 2015, 4, 251–262.
- Ravichandran, S.; Vimala, R. Solid State and Submerged Fermentation for the Production of Bioactive Substances: A Comparative Study. Int. J. Sci. Nat. 2012, 3, 480–486.
- Yamuna Rani, K.; Ramachandra Rao, V.S. Control of Fermenters—A review. Bioprocess Eng. 1999, 21, 77–88.
- Linder, T. Making the Case for Edible Microorganisms as an Integral Part of a More Sustainable and Resilient Food Production System. Food Sec. 2019, 11, 265–278.
- FAO. World Food and Agriculture–Statistical Yearbook 2021; FAO Statistical Yearbook–World Food and Agriculture; FAO: Rome, Italy, 2021; ISBN 978-92-5-134332-6.
- WHO. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8.
- Balali, G.I.; Yar, D.D.; Afua Dela, V.G.; Adjei-Kusi, P. Microbial Contamination, an Increasing Threat to the Consumption of Fresh Fruits and Vegetables in Today’s World. Int. J. Microbiol. 2020, 2020, e3029295.
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical Composition of Some Selected Fruit Peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21.
- Ibrahim, U.K.; Kamarrudin, N.; Suzihaque, M.U.H.; Hashib, S.A. Local Fruit Wastes as a Potential Source of Natural Antioxidant: An Overview. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Miri, Malaysia, 1–3 December 2016; Volume 206, p. 012040.
- Sharma, R.; Oberoi, H.S.; Dhillon, G.S. Chapter 2-Fruit and Vegetable Processing Waste: Renewable Feed Stocks for Enzyme Production. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Dhillon, G.S., Kaur, S., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 23–59. ISBN 978-0-12-802392-1.
- Abdullah; Mat, H.B. The Characteristic of Pineapple Waste from Canning Industry. Adv. Sci. Lett. 2017, 23, 5691–5693.
- Murakonda, S.; Dwivedi, M. Powders from Fruit Waste. In Food Powders Properties and Characterization; Ermiş, E., Ed.; Food Engineering Series; Springer: Cham, Switzerland, 2021; pp. 155–168. ISBN 978-3-030-48908-3.
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Chapter Five-Bioactive Potential of Fruit and Vegetable Wastes. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 157–225.
- Mahato, N.; Sharma, K.; Sinha, M.; Dhyani, A.; Pathak, B.; Jang, H.; Park, S.; Pashikanti, S.; Cho, S. Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation. Processes 2021, 9, 220.
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit By-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716.
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812.
- Monspart-Sényi, J. Fruit Processing Waste Management. In Handbook of Fruits and Fruit Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 315–331. ISBN 978-1-118-35253-3.
- Panda, S.K.; Ray, R.C.; Mishra, S.S.; Kayitesi, E. Microbial Processing of Fruit and Vegetable Wastes into Potential Biocommodities: A Review. Crit. Rev. Biotechnol. 2018, 38, 1–16.
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules 2018, 23, 2560.
- Spalvins, K.; Zihare, L.; Blumberga, D. Single Cell Protein Production from Waste Biomass: Comparison of Various Industrial by-Products. Energy Procedia 2018, 147, 409–418.
- De Gregorio, A.; Mandalari, G.; Arena, N.; Nucita, F.; Tripodo, M.M.; Lo Curto, R.B. SCP and Crude Pectinase Production by Slurry-State Fermentation of Lemon Pulps. Bioresour. Technol. 2002, 83, 89–94.
- Sadhu, S.D.; Garg, M.; Kumar, A. 4-Major Environmental Issues and New Materials. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 77–97. ISBN 978-0-12-811033-1.
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of Agro-Industrial Wastes in Solid-State Fermentation Processes; IntechOpen: London, UK, 2012; ISBN 978-953-51-0253-3.
- Saheed, O.K.; Jamal, P.; Karim, M.I.A.; Alam, M.Z.; Muyibi, S.A. Utilization of Fruit Peels as Carbon Source for White Rot Fungi Biomass Production under Submerged State Bioconversion. J. King Saud Univ.-Sci. 2016, 28, 143–151.
- Saheed, O.K.; Jamal, P.; Kari, M.I.A.; Alam, Z.; Muyibi, S.A. Cellulolytic Fruits Wastes: A Potential Support for Enzyme Assisted Protein Production. J. Biol. Sci. 2013, 13, 379–385.
- Umesh, M.; Thazeem, B.; Preethi, K. Valorization of Pineapple Peels through Single Cell Protein Production Using Saccharomyces cerevisiae NCDC 364. Appl. Food Biotechnol. 2019, 6, 255–263.
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318.
- Ani, P.N.; Abel, H.C. Nutrient, Phytochemical, and Antinutrient Composition of Citrus maxima Fruit Juice and Peel Extract. Food Sci. Nutr. 2018, 6, 653–658.
- Dias, P.G.I.; Sajiwanie, J.W.A.; Rathnayaka, R.M.U.S.K. Chemical Composition, Physicochemical and Technological Properties of Selected Fruit Peels as a Potential Food Source. Int. J. Fruit Sci. 2020, 20, S240–S251.
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit Peel Waste: Characterization and Its Potential Uses. Curr. Sci. 2017, 113, 444–454.
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged Citric Acid Fermentation on Orange Peel Autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387.
- Orozco, R.S.; Hernández, P.B.; Morales, G.R.; Núñez, F.U.; Villafuerte, J.O.; Lugo, V.L.; Ramírez, N.F.; Díaz, C.E.B.; Vázquez, P.C. Characterization of Lignocellulosic Fruit Waste as an Alternative Feedstock for Bioethanol Production. BioResources 2014, 9, 1873–1885.
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, Hemicelluloses, Lignin and Ash Content of Some Organic Materials and Their Suitability for Use as Paper Pulp Supplements. Bioresour. Technol. 2007, 98, 296–301.
- Mondal, A.K.; Sengupta, S.; Bhowal, J.; Bhattacharya, D.K. Utilization of Fruit Wastes in Producing Single Cell Protein. Int. J. Sci. Environ. 2012, 1, 430–438.
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116.
- Adoki, A. Factors Affecting Yeast Growth and Protein Yield Production from Orange, Plantain and Banana Wastes Processing Residues Using Candida Sp. Afr. J. Biotechnol. 2008, 7, 290–295.
- Malav, A.; Dube, P. Single Cell Protein Production Using Various Microbial Mass: A Review. IJAR 2017, 5, 2190–2194.
- Kandari, V.; Gupta, S. Bioconversion of Vegetable and Fruit Peel Wastes in Viable Product. J. Microbiol. Biotechnol. Res. 2012, 2, 308–312.
- Adedayo; Ajiboye, E.A.; Akintunde, J.K.; Odaibo, A. Single Cell Proteins: As Nutritional Enhancer. Adv. Appl. Sci. Res. 2011, 2, 396–409.
- Bacha, U.; Nasir, M.; Khalique, A.; Anjum, A.; Jabbar, M. Comparative Assessment of Various Agro-Industrial Wastes for Saccharomyces cerevisiae Biomass Production and Its Quality Evaluation as Single Cell Protein. J. Anim. Plant Sci. 2011, 21, 844–849.
- Akanni, G.; Ntuli, V.; Preez, D. Cactus Pear Biomass, a Potential Lignocellulose Raw Material for Single Cell Protein Production (SCP): A Review. Int. J. Curr. Microbiol. App. Sci 2014, 3, 171–197.
- Khan, M.; Khan, S.; Zafar, A.; Tanveer, A. Production of Single Cell Protein from Saccharomyces cerevisiae by Utilizing Fruit Wastes. Nanobiotechnica Univers. 2010, 1, 127–132.
- Thiviya, P.; Kapilan, R.; Madhujith, T. Bioconversion of Fruit Wastes of Papaya, Watermelon, and Banana into Single Cell Protein Production. Trop. Agric. Res. 2021, 32, 503–514.
- Rages, A.A.; Haider, M.M. Alkaline Hydrolysis of Olive Fruits Wastes for the Production of Single Cell Protein by Candida lipolytica. Biocatal. Agric. Biotechnol. 2021, 33, 101999.
- Nigam, J.N. Single Cell Protein from Pineapple Cannery Effluent. World J. Microbiol. Biotechnol. 1998, 14, 693–696.
- Rosma, A.; Ooi, K.I. Production of Candida utilis Biomass and Intracellular Protein Content: Effect of Agitation Speed and Aeration Rate. MJM 2006, 2, 15–18.
- Munawar, R.; Irfan, M.; Nadeem, M.; Syed, Q.; Siddique, Z. Biosynthesis of Single Cell Biomass of Candida Utuilis by Submerged Fermentation. Pak. J. Sci. 2010, 62, 1–5.
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-González, R. Production of Single Cell Protein from Orange Peel Residues by Candida utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298.
- Somda, M.K.; Nikiema, M.; Keita, I.; Mogmenga, I.; Kouhounde, S.H.S.; Dabire, Y.; Coulibaly, W.H.; Taale, E.; Traore, A.S. Production of Single Cell Protein (SCP) and Essentials Amino Acids from Candida utilis FMJ12 by Solid State Fermentation Using Mango Waste Supplemented with Nitrogen Sources. AJB 2018, 17, 716–723.
- Jiru, T.M.; Melku, B. Single Cell Protein Production from Torula Yeast (Cyberlindnera Sp.) Using Banana Peel Hydrolysate. J. Adv. Microbiol. 2018, 13, 1–7.
- Ziino, M.; Lo Curto, R.B.; Salvo, F.; Signorino, D.; Chiofalo, B.; Giuffrida, D. Lipid Composition of Geotrichum candidum Single Cell Protein Grown in Continuous Submerged Culture. Bioresour. Technol. 1999, 67, 7–11.
- Stabnikova, O.; Wang, J.-Y.; Bo Ding, H. Joo-HwaTay Biotransformation of Vegetable and Fruit Processing Wastes into Yeast Biomass Enriched with Selenium. Bioresour. Technol. 2005, 96, 747–751.
- Abarshi, M.M.; Mada, S.B.; Amin, M.I.; Salihu, A.; Garba, A.; Mohammad, H.A. Effect of Nutrient Supplementation on Single Cell Protein Production from Watermelon and Pineapple Peels. Niger. J. Basic Appl. Sci. 2017, 25, 130–136.
- Aruna, T.E.; Aworh, O.C.; Raji, A.O.; Olagunju, A.I. Protein Enrichment of Yam Peels by Fermentation with Saccharomyces cerevisiae (BY4743). Ann. Agric. Sci. 2017, 62, 33–37.
- Mensah, J.K.M.; Twumasi, P. Use of Pineapple Waste for Single Cell Protein (SCP) Production and the Effect of Substrate Concentration on the Yield. J. Food Process Eng. 2017, 40, e12478.
- Mujdalipah, S.; Putri, M.L. Utilization of Pineapple Peel and Rice Washing Water to Produce Single Cell Proteins Using Saccharomyces cerevisiae. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 9–10 October 2019; Volume 472, p. 012029.
- Nurmalasari, A.; Maharani, S. Addition of Carbon Sources to Pineapple Waste Media in the Production of Single Cell Protein Biomass Saccharomyces Cerevisiae. J. Ris. Biol. Dan Apl. 2020, 2, 70–76.
- Umesh, M.; Priyanka, K.; Thazeem, B.; Preethi, K. Production of Single Cell Protein and Polyhydroxyalkanoate from Carica papaya Waste. Arab. J. Sci. Eng. 2017, 42, 2361–2369.
- Muniz, C.E.S.; Santiago, Â.M.; Gusmão, T.A.S.; Oliveira, H.M.L.; de Sousa Conrado, L.; de Gusmão, R.P. Solid-State Fermentation for Single-Cell Protein Enrichment of Guava and Cashew by-Products and Inclusion on Cereal Bars. Biocatal. Agric. Biotechnol. 2020, 25, 101576.
- Azam, S.; Khan, Z.; Bashir, A.; Khan, I.; Ali, J. Production of Single Cell Protein from Orange Peels Using Aspergillus niger and Saccharomyces cerevisiae. Glob. J. Biotechnol. Biochem. 2014, 9, 14–18.
- Hamdy, H.S. Production of Mini-Food by Aspergillus niger, Rhizopus oryzae and Saccharomyces cerevisiae Using Orange Peels. Rom. Biotechnol. Lett. 2013, 18, 7929–7946.
- Rashad, M.M.; Moharib, S.A.; Jwanny, E.W. Yeast Conversion of Mango Waste or Methanol to Single Cell Protein and Other Metabolites. Biol. Wastes 1990, 32, 277–284.
- Oshoma, C.E.; Eguakun-Owie, S.O. Conversion of Food Waste to Single Cell Protein Using Aspergillus niger. J. Appl. Sci. Environ. Manag. 2018, 22, 350–355.
- Yabaya, A.; Ado, S.A. Mycelial Protein Production by Aspergillus niger Using Banana Peels. Sci. World J. 2008, 3, 9–12.
- Kamal, M.; Ali, M.; Shishir, M.R.I.; Saifullah, M.; Haque, M.; Mondal, S.C. Optimization of Process Parameters for Improved Production of Biomass Protein from Aspergillus Niger Using Banana Peel as a Substrate. Food Sci. Biotechnol. 2019, 28, 1693–1702.
- Bind, A.; Kumar, M.; Singh, D. Optimization of SCP Production of Aspergillus niger Using Different Fruit Peels-Indian Journals. Int. J. Bioinform. Biol. Sci. 2013, 1, 1–8.
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; de la Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of Agro Industrial Wastes as Immobilization Carrier for Solid-State Fermentation. Ind. Crops Prod. 2009, 30, 24–27.
- Jaganmohan, P.; Daas, B.P.; Prasad, S.V. Production of Single Cell Protein (SCP) with Aspergillus terreus Using Solid State Fermentation. Eur. J. Biol. Sci. 2013, 5, 38–43.
- Scerra, V.; Caridi, A.; Foti, F.; Sinatra, M.C. Influence of Dairy Penicillium Spp. on Nutrient Content of Citrus Fruit Peel1Contribution from the Ministry of Scientific Research and Technology–Research Fund 60%: M. C. Sinatra.1. Anim. Feed. Sci. Technol. 1999, 78, 169–176.
- Khan, M.; Khan, S.S.; Ahmed, Z.; Tanveer, A. Production of Fungal Single Cell Protein Using Rhizopus Oligosporus Grown on Fruit Wastes. Biol. Forum 2009, 1, 26–28.
- Ahmadi, F.; Zamiri, M.J.; Khorvash, M.; Banihashemi, Z.; Bayat, A.R. Chemical Composition and Protein Enrichment of Orange Peels and Sugar Beet Pulp after Fermentation by Two Trichoderma Species. Iran. J. Vet. Res. 2015, 16, 25–30.
- Mahan, K.M.; Le, R.K.; Wells, T., Jr.; Anderson, S.; Yuan, J.S.; Stoklosa, R.J.; Bhalla, A.; Hodge, D.B.; Ragauskas, A.J. Production of Single Cell Protein from Agro-Waste Using Rhodococcus Opacus. J. Ind. Microbiol. Biotechnol. 2018, 45, 795–801.
- Patel, N.; Patel, A.; Patel, H.; Patel, M.; Patel, U. Production of Single Cell Protein from Mix Fruits Waste Using Lactobacillus. Int. J. Pharm. Biol. Sci. 2019, 9, 164–168.
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1.
- Domingues, R.; Bondar, M.; Palolo, I.; Queirós, O.; de Almeida, C.D.; Cesário, M.T. Xylose Metabolism in Bacteria—Opportunities and Challenges towards Efficient Lignocellulosic Biomass-Based Biorefineries. Appl. Sci. 2021, 11, 8112.
- Sandle, T. 22-Microbiological Challenges to the Pharmaceuticals and Healthcare. In Pharmaceutical Microbiology; Sandle, T., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 281–294. ISBN 978-0-08-100022-9.
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224.
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-Industrial Lignocellulosic Biomass a Key to Unlock the Future Bio-Energy: A Brief Review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173.
- Kalaichelvan, P.T.; Arulpandi, I. Bioprocess Technology; MJP Publishers: Chennai, India, 2019; ISBN 978-81-8094-032-3.
- Chen, H. Chemical Composition and Structure of Natural Lignocellulose. In Biotechnology of Lignocellulose: Theory and Practice; Chen, H., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 25–71. ISBN 978-94-007-6898-7.
More
