Cassava is one of the most important sources of energy. To meet the growing demand, genetic improvement is of utmost importance. Its cross-pollinating nature limits the opportunity of exploitation of hybrid vigour and demands the development of homozygous lines through doubled-haploid technologies. The problems in callus mediated embryogenesis such as longer processing time and genetically unstable nature can be overcome by direct embryogenesis. Conditions to produce embryos directly from microspores in cultured anthers were optimized. The optimum stress pre-treatment condition was 40°C for 6 h after culturing the anthers in to the induction medium. For pro-embryo formation, 2% sucrose and 5 mg/l 2,4-Dichlorophenoxyacetic acid (2,4-D) or 1 mg/l 1-Naphthaleneacetic acid were optimum. Globular embryos were formed by sub-culturing pro-embryos into the medium with 0.5 mg/l 2,4-D and 5 mg/l 6-Benzylaminopurine after two weeks of culturing. Light microscopy of cultured anthers demonstrated the formation of multicellular structures and their further development into pro-embryos. Microscopic studies showed pro-embryos emerging through the damaged anther wall. Mono-allelic banding in Simple Sequence Repeat (SSR) analysis indicated homozygous or haploid state in some of the originated embryos. The conditions optimized in this study were effective in early development of direct embryos after two weeks of culture initiation. This is the first report of formation of direct embryos in cultured anthers of cassava.
- Manihot esculenta
- Cassava
- Light micrographs
- Scanning Electron Micrographs
- Proembryos
- Anther culture
- Androgenesis
1. Morphological Aspects of Anthers in Culture
Stereo microscopic observations showed that fresh anthers had a smooth anther wall surface in greenish color (Figure 1a). After one week of culturing, all anthers turned to brownish. An enlargement of anthers was observed in some anthers, while the others remained unchanged (Figure 1b). With time, the multicellular structures at the early stage of embryogenesis were visible through the translucent, probably dead, anther wall (Figure 1c). Such proembryos could be observed most of the time after two weeks of culture initiation but, also, up to eight weeks later, indicating that the potential for embryogenesis exists for a considerable period of time. Later, proembryos were visible outside the damaged anther wall (Figure 1d). They were round and had a fragile translucent appearance and a smooth surface.

Figure 1. Morphological aspects of anther-derived proembryos of Manihot esculenta var. Kirikawadi. (a) Fresh anthers isolated from the male flower buds. (b) Enlarged anthers. Note the nonresponsive ones showing anther browning. (c) Visualizing a multicellular structure (arrow) enclosed in the anther lobe. (d) Translucent proembryo with the smooth surface emerging through the anther lobe (Al). (e) A proembryo converted into ivory color. (f) Close view of proembryos emerging through the anther wall. Arrows indicate the remnants of the anther wall (Aw) after emergence of the embryo through the broken anther wall. Bars: (a,b,f) 1 mm, (c) 670 µm, (d) 500 µm and (e) 600 µm.
By transferring anthers with proembryos of variable sizes onto a solid medium with a low auxin:cytokinin ratio (1:10), the globular embryos converted into a white, opaque appearance (Figure 1e). Most of the responsive anthers gave rise to multiple embryos (Figure 1f), whereas some gave rise to the secondary embryos as well.
2. Scanning Electron Microscopic Observation
Observations on fresh (Figure 2a) and cultured anthers revealed that embryos popped out from a cracked anther wall. Proembryos just emerging through the anther wall clearly revealed the absence of any physical attachment to the anther wall cells (Figure 2b,c). Proembryos and embryos were characterized by globular shapes with a smooth outer surface (Figure 2d).
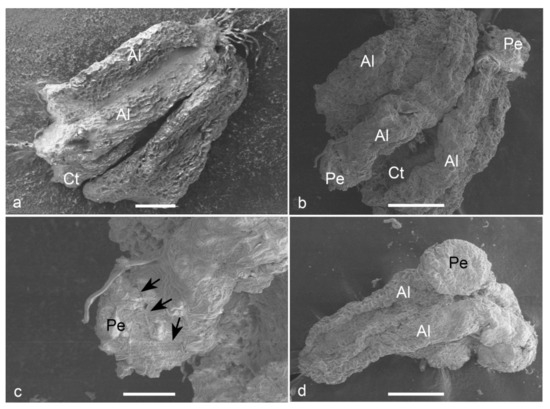
Figure 2. Scanning electron micrographs (SEM) of the anther-derived proembryos of Manihot esculenta var. Kirikawadi. (a) A fresh anther (Al—anther lobe and Ct—connective tissue). (b) The proembryos (Pe) emerging through the anther wall. (c) Close view of a proembryo emerging from the anther lobe of left side in b. Black arrows indicate the broken anther wall edge. (d) Globular embryo with a smooth surface. Bars: (a) 100 µm, (b,d) 200 µm and (c) 80 µm.
