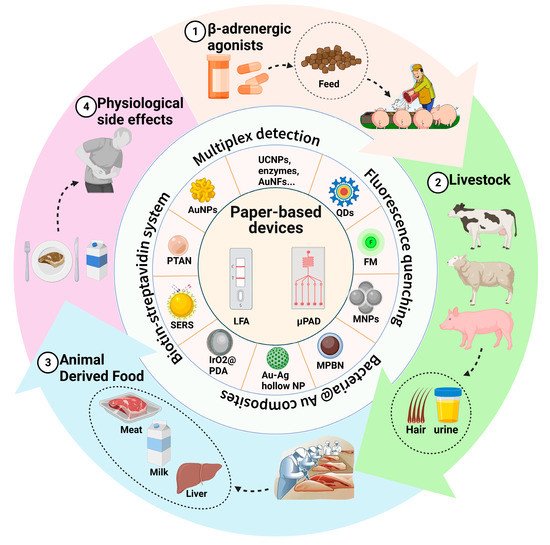
The illegal use of β-adrenergic agonists during livestock growth poses a threat to public health; the long-term intake of this medication can cause serious physiological side effects and even death. Therefore, rapid detection methods for β-adrenergic agonist residues on-site are required. Traditional detection methods such as liquid chromatography have limitations in terms of expensive instruments and complex operations. In contrast, paper methods are low cost, ubiquitous, and portable, which has led to them becoming the preferred detection method in recent years. Various paper-based fluidic devices have been developed to detect β-adrenergic agonist residues, including lateral flow immunoassays (LFAs) and microfluidic paper-based analytical devices (μPADs).
1. Introduction
Food safety and food control are of increasing concern for food markets around the world. Animal-derived food provides a substantial protein source for humans and constitutes a notable proportion of many peoples’ diets. To maintain animal husbandry practices and help to develop the industry, veterinary drugs are widely used for the prevention and treatment of diseases, as well as for the promotion of growth
[1]. Unfortunately, the uncontrolled use of veterinary drugs, especially synthesized β-adrenergic agonists known as “lean meat powder”, has caused many public health problems
[2] and is now an issue of concern around the world.
The synthesized β-adrenergic agonist family (“lean meat powder”) includes clenbuterol (CLE), ractopamine (RAC), salbutamol (SAL), terbutaline, and clorprenaline, and they have been widely used to treat lung disease and asthma in humans. Furthermore, members of this family can also be used to improve fat decomposition and conversion and are therefore used as feed additives to accelerate growth and increase muscle in animals
[3][4][3,4]. However, long-term or high-dose intake of residual β-adrenergic agonists through meat products can cause serious and harmful physiological side effects such as myalgia, dizziness, tachycardia, nervousness, and even death
[5]. β-adrenergic agonists can accumulate in animal tissues as they do not readily decompose
[6]. Due to this, β-adrenergic agonists are currently prohibited as feed additives in China and most European and American countries; however, several β-adrenergic agonists continue to be illegally added for economic benefits in many countries and regions
[7][8][7,8]. For example, there was a clenbuterol (CLE) event in China in mid-March 2011 which sparked serious debate about food safety in relation to β-adrenergic agonists
[9]. On 19 March 2021, the Ministry of Agriculture and Rural Affairs of the People’s Republic of China issued the “Notice on Launching a Special Rectification Action” for “lean meat powder” to severely crack down on its illegal use.
Due to the potential risks of β-adrenergic agonist residues on human health, and considering the need to monitor their illegal use, sensitive wide-ranged screening and convenient assays are required to allow detection on-site. In recent years, various analytical methods have been developed for the detection of β-adrenergic agonists, including liquid chromatography (LC)
[10][11][10,11], gas chromatography-mass spectrometry (GC-MS)
[12], LC-tandem mass spectrometry (LC-MS/MS)
[13], GC-MS/MS
[14], and capillary electrophoresis
[10]. However, these methods are costly, laborious, and time-consuming, which hinders their use as on-site assays. In contrast, enzyme-linked immunosorbent assay (ELISA) is a sensitive and relatively simple procedure, yet it has a high false positive rate and is difficult to use on-site.
Paper has become a simple and reliable platform for analytical instruments, and one of the most well-known applications is the lateral flow immunoassay (LFA). Due to its unique advantages, including rapidity, portability, and simplicity, LFAs have been widely used as tests for clinical diagnosis
[15][16][15,16], infectious diseases
[17], and the detection of chemical contaminants
[18]. LFAs combine immune-specific recognition and sensitive nano-signal characteristics, creating an excellent tool for use in point-of-care tests (POCT) for food safety. Microfluidic paper-based analytical devices (μPADs) are the newest generation of “lab-on-a-chip” devices. Since the first μPAD was released in 2007
[19], they have experienced rapid development. Using paper as a substrate for microfluidic devices has the advantages of low cost, wide availability, and simple fabrication; more importantly, externally powered equipment need not manipulate the fluid and conduct biochemical reactions. Although μPADs are still in the preliminary development stage for the detection of β-adrenergic agonists, satisfactory results have been achieved (
Figure 1). Vertical flow immunoassays (VFAs) offer an improvement on the performance of LFAs due to their obvious advantages in comparison, such as shorter detection time and stronger multiplexing capability
[20] and, consequently, they have the potential to be used for the high-throughput and ultra-sensitive on-site detection of “lean meat powder.”
Figure 1. Illegal addition of β-adrenergic agonists to livestock feed poses a huge threat to public health. Various paper-based fluidic devices have been developed to detect β-adrenergic agonist residues in animal-derived food, hair, and urine from livestock, including lateral flow immunoassays (LFAs) and microfluidic paper-based analytical devices (μPADs). During the past decades, researchers have improved the detection performance of devices by developing novel labeling and detection strategies.
2. LFA Formats and Principles
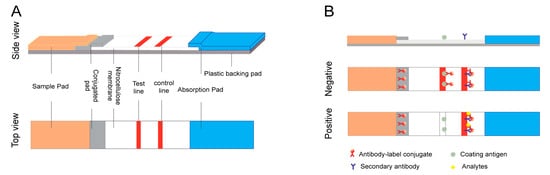
A typical LFA device contains four sections (sample pad, conjugate pad, nitrocellulose membrane, and absorbance pad) which are laminated in an orderly manner onto a sheet of plastic backing (
Figure 2A). Various formats are possible depending on the type of target analyte
[21], but sandwich and competitive assays are the two standard formats most frequently used.
Figure 2. Schematic illustration of the lateral flow immunoassay (LFA) test strip. (
A) Typical composition of an LFA test strip, and (
B) principles of competitive LFA testing.
2.1. Sandwich Format
Sandwich assays are typically used for high molecular weight molecules or targets with multiple antigenic sites such as proteins and bacteria. Generally, this system uses a specific antibody pair, as the test line is coated with the unlabeled antibody, and the detected antibody is used to bind the signal label. When the sample is added to the test strip, the specific antibody reacts with the labeled antibody–target complex to form a clear test line through sample migration. The response detected in the test zone is directly proportional to the number of targets in the sample. The mixture then passes through the capture zone, where both the unbound and bound analytes bind to the capture antibody
[22][23][22,23].
2.2. Competitive Format
Competitive assays are employed most often when testing low molecular weight analytes or when presenting a single antigenic determinant. For this format, the analyte–protein complex is immobilized on the test line, competing with the target in the sample to conjugate the labeled antibody. The response detected on the test line is negatively proportional to the amount of the target in the sample. Another specific antibody bound to the control line allows for the capture of excess antibody complex. Therefore, a band color appears in the control line regardless of the presence of the target analyte, confirming that the test was run correctly (
Figure 2B)
[22][23][22,23].
The test strip is a one-step procedure and the liquid sample to be analyzed is placed on the sample pad. All membrane pads are usually made with nitrocellulose. The reagent membrane comprises the immobilized specific antibodies and the labeled antibodies. With the addition of the sample, the reacting molecules are solubilized and combined with the expected metrics in the sample. Then, capillary action directs the fluid mixture to the reaction membrane
[21].
3. Applications for Detecting β-Adrenergic Agonist Residues
β-adrenergic agonists are a group of synthetic phenethanolamine compounds, and according to their different aromatic ring structures they can be separated into three categories: aniline, phenol, and resorcinol
[24]. LFA technology has been adequately developed to detect β-adrenergic agonists. As they are low molecular mass compounds, competitive assays should be used, the principles of which were described in
SectiSection 2 o
n 2 of this
entryreview (2. LFA formats and principles). If the analyte is not present in the sample, then the labeled antibody will be caught by the bovine serum albumin (BSA)-analyte and immobilized on the membrane to form a clear test line with negative results; if the analyte is present in the sample exceeding the lower detectable concentration, it competes with the BSA-analyte immobilized on the test line and binds to the finite amount of labeled antibody, leading to an invisible test line with positive results. Published reports on LFA applications in this field are summarized in
Table 1.
Table 1.
Summary of the analytical performance of traditional paper-based LFAs for the detection of β-adrenergic agonists.
| Analyte |
Label |
Assay Format |
Sample |
Assay Time |
LOD |
Reference |
| CLE 1 and RAC 3 |
AuNPs 2 |
Competitive LFA |
Swine urine |
5 min |
0.1 ± 0.01 ng/mL |
[25] |
| CLE |
AuNPs |
Competitive LFA |
Swine livers |
10 min |
NA |
[26] |
| CLE |
AuNPs |
Competitive LFA |
Swine urine |
10 min |
3 ng/mL |
[27] |
| CLE |
AuNPs |
Competitive LFA |
Swine urine |
10 min |
0.1 ng/mL |
[28] |
| CLE, RAC, SAL 4 |
AuNPs |
Competitive LFA |
Swine urine |
10 min |
0.5 ng/mL |
[29] |
| CLE |
AuNPs |
Competitive LFA |
Swine urine |
10 min |
220 pg/mL |
[30] |
| SAL |
AuNPs |
Competitive LFA |
Swine urine |
10 min |
80 ng/mL |
[31] |
| RAC |
AuNPs |
Competitive LFA |
Swine urine |
5 min |
0.1 ng/mL |
[32] |
| SAL |
AuNPs |
Competitive LFA |
Meat and milk |
10 min |
meat: 4.0 ng/g
milk: 3.0 ng/g |
[33] |
| RAC |
AuNPs |
Competitive LFA |
Swine urine |
45 min |
0.13 ng/mL |
[34] |
| CLE |
SeNPs 5 |
Competitive LFA |
Swine urine |
/ |
3 ng/mL |
[35] |
| RAC and SAL |
SeNPs |
Competitive LFA |
Swine urine |
5 min |
RAC: 1 ng/mL
SAL: 3 ng/mL |
[36] |
| CLE and RAC |
SiNPs 6 |
Competitive LFA |
/ |
10 min |
CLE: 3 ng/mL
RAC: 2 ng/mL |
[37] |
| CLE |
SiNPs |
Competitive LFA |
PBS, urine, and pork |
10 min |
PBS: 3 ng/mL
urine: 6 ng/mL
pork: 5 ng/mL |
[38] |
| Zilpaterol |
AuNPs |
Competitive LFA |
Feed |
10 min |
20 ng/g |
[39] |
| PA 7 |
AuNPs |
Competitive LFA |
Swine urine |
10 min |
0.188 ng/mL |
[40] |
| Clorprenaline |
AuNPs |
Competitive LFA |
Swine urine |
3–5 min |
0.104 ng/mL |
[41] |
| Clorprenaline |
AuNPs |
Competitive LFA |
Swine urine |
9 min |
0.15 ng/mL |
[42] |