Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Giulia Poletto and Version 2 by Conner Chen.
Radiolabeled liposomes have attracted new interest as probes to identify the most suitable patients for treatment with liposomal formulations of common chemotherapeutics. The use of ligands for the delivery of radiotherapeutics to a specific target is still the most appealing strategy for treating tumors. The most appropriate ligand can be identified by virtually simulating its interaction with the receptor. All strategies showed great potential for use in targeted radionuclide therapy, but they also have numerous drawbacks.
- targeted radionuclide therapy
- liposomes
- avidin–biotin
- docking
1. Introduction
The goal of cancer therapy is to target and destroy tumor cells without damaging healthy tissues, but chemotherapy and external beam radiotherapy (EBRT)—the main alternatives to surgical treatment—are often nonspecific or associated with toxicity [1]. Many efforts have consequently been made to find ways to deliver cancer treatments more precisely. One example is targeted radionuclide therapy (TRT), a method whereby radionuclides are carried to tumor cells by molecules with a high affinity for the target [2]. Delivering radionuclides more effectively brings many advantages. For a start, the dose of isotope administered can be modulated, reducing patients’ exposure to radiation and the costs of the treatment. Second, a high affinity for the target can reduce background activity, thereby improving imaging quality and drug tolerability. Third, the opportunity to obtain information about a drug’s biodistribution using a diagnostic agent enables the planning of patient-specific treatments. To obtain the abovementioned advantages, the tracer is administered, at first, labeled with positron or γ-emitting radionuclides (e.g., 18F, 68Ga, or 111In) to see the areas where the tracer is deposited, identify any off-target uptake [3], and predict the dose absorbed by the tumor. Then the same tracer is administered again after labeling with β-emitting radionuclides (e.g., 90Y, 177Lu, or 131I).
Alpha-emitting radionuclides and Auger electron emitters can also be used for therapy, but β-emitting radionuclides are considered ideal for treating large tumors. This is because their long-range radiation can affect neighboring cells, as well as those being targeted (crossfire effect). Alpha-emitters are short-range, high-energy emitters more suitable for treating micrometastases and blood or bone marrow malignancies. Auger electron emitters are better suited to targeting single cells [1].
The choice of radiopharmaceutical agent is of primary importance in TRT. Carrier molecules should have a high affinity and specificity for the target, they should not be toxic or immunogenic, they should be stable before and after administration, they should be capable of binding a variety of radionuclides effectively, and they should be readily available at low cost [2].
Monoclonal antibodies (MoAbs) are among the most often used targeting agents because they can recognize a specific target and be bound directly to a radionuclide. They also have several drawbacks, however, such as a large size and slow kinetics [2]. The fact that some radionuclides decay rapidly has made it necessary to reduce the circulation time of MoAbs, and this has prompted the development of engineered antibody fragments, such as single-domain antibodies, diabodies, minibodies, protein scaffolds, and more complex specific antibodies [4]. Smaller antibodies have better pharmacokinetics and a good tumor penetration. They also have dimensions below the renal filtration cutoff; hence, they can be cleared through the kidneys, which are less radioresistant than the liver (the main site of MoAb accumulation) [4].
The high expression of peptide receptors on the surface of tumors means that peptide analogs are also good targeting agents [2]. Somatostatin receptors have been used as targets for over 20 years [5], especially for the treatment of neuroendocrine tumors. The most often used somatostatin analogs are dodecanetetraacetic acid phenylalanine-1 tyrosine 3-octreotide (DOTA-TOC) and dodecanetetraacetic acid tyrosine 3-octreotate (DOTA-TATE). They are labeled with 90Y and 177Lu for treatment purposes, and with 68Ga or 111In or 99mTc for pretreatment imaging. The advantages of using peptide analogs include a well-established conjugation chemistry, an efficient penetration in solid tumors, and lower production costs [1].
Small molecules like hormones, steroids, and neurotransmitters that are internalized by specific receptors can also be used as targeting agents. An example is metaiodobenzylguanidine (MIBG), a structural analog of the neurotransmitter norepinephrine, which can be labeled with 131I or 123I for use in treating or imaging in patients with relapsing or refractory neuroblastoma, neuroendocrine tumors, or medullary thyroid cancers [1].
Despite this variety of promising targeting agents, the clinical efficiency of TRT remains low for solid tumors because the targeting agents become distributed mainly in the outer part of the tumor mass, with less radiation reaching the inner part [2].
2. Liposomes
Liposomes are nanosized vesicles consisting of a lipid bilayer that can also contain cholesterol. They have an aqueous core and can be filled with either hydrophobic drugs (encapsulated in the bilayer) or hydrophilic drugs (encapsulated in the aqueous core). By means of a lipid chain, the main molecules (e.g., drugs and targeting agents) can also be linked to the membrane surface during liposome manufacture or via post-synthesis [6]. Embedding drugs in liposomes improves their properties, achieving a better biodistribution and a lower toxicity [7], and that is why these liposomal vesicles are often used for conventional drug delivery. Liposomes were found to accumulate at tumor sites thanks to the enhanced permeability and retention (EPR) effect. They are easily taken up by the reticuloendothelial system (RES), however [7], consequently accumulating in organs such as the liver and spleen. To avoid their rapid clearance, polyethylene glycol (PEG) chains can be conjugated to the liposome surface to extend their circulation time and enhance their accumulation at tumor sites [7]. Studies on liposomes as drug delivery agents for use with radionuclides began in the last decade and led to the synthesis of a liposomal imaging tool called Vescan, which was never commercialized as it proved unable to detect tumors [8][9][36,37]. Attention has now shifted from the radiolabeling of empty liposomes to the radiolabeling of liposomal formulations of conventional chemotherapeutics. The aims of these contenttudies on this topic are to identify the pharmacokinetic (PK) properties of liposomal formulations once injected in vivo, and to establish which patients will better respond to this therapy. Although liposomes are not a perfect example of a TRT, examining progress made in research on these vesicles can probably help peopleus to better understand their potential future uses. Here areWe selected 16 articles on the radiolabeling of liposomal formulations of conventional chemotherapeutics: nine studies dealing with the pharmacokinetics of different liposomal formulations, and seven studies dealing with the patients’ different responses to the administration of liposomal formulations of chemotherapeutics. The content of these studies is summarized in Table 1. The pharmacokinetic properties of liposomes may be influenced by the presence of a targeting agent on their surface. To give an example, Du and coworkers [10][13] synthesized liposomes functionalized with MoAbs against programmed cell death-1 (PD-1), a receptor selectively expressed in triple-negative breast cancer. These liposomes were then filled with doxorubicin (DOX) and dual-labeled with a fluorophore (IRDye800WC) and a radionuclide (64Cu). They proved better able to target and to treat the tumor due to the simultaneous effect of the MoAbs against PD-1 both as a targeting agent for liposomes and as an adjuvant immunotherapy for doxorubicin. Alongside the presence of the targeting agent, the composition of a liposome may also influence its pharmacokinetic properties. Silva and coworkers [11] demonstrated that long-circulating, pH-sensitive liposomes (SpHL) containing [99mTc] DOX accumulated more in the tumor and were less active in the spleen and liver than liposomes that were not pH-sensitive. That said, Monteiro and coworkers [12][16] noted that the presence of folate on the surface of SpHL (filled with paclitaxel) may lead to an even more sustained and higher tumor-to-muscle ratio than in the case of nonfunctionalized liposomes. In addition to the presence of a targeting agent, other physical characteristics may enhance liposome delivery. For instance, Yang and coworkers [7] were able to obtain a good tumor brain delivery of their liposomal formulation of DOX, with a high tumor-to-contralateral brain ratio. They associated the presence of a targeting agent (AP-1, a peptide capable of binding IL-4 receptor) with the focused ultrasound technique, which enables a temporarily disruption of the blood–brain barrier. Reversible electroporation may also enhance delivery to the tumor, with or without any targeting agent on the liposome’s surface; this technique enhances vascular permeability, altering the EPR effect and, thus, leading to a greater liposome deposition at the tumor site [13][17]. To better study liposome distribution, the fluorescence technique can be associated with imaging, using positron emission tomography (PET), as in the earlier-mentioned work by Du et al. [10][13]. Li and coworkers [6] also succeeded in developing liposomes suitable for this application; their formulation could be labeled with the fluorophore IRDye-DSPE and the radionuclides 99mTc, 186/188Re, or 64Cu thanks to the presence of DOTA on the liposome’s surface [6]. Luo and coworkers [14][15] demonstrated that adding porphyrin phospholipid to the liposome’s bilayer may also be useful for the development of liposomal vesicles suitable for multimodality imaging. Double radiolabeling is another way to obtain more information about the final target of both the liposome and the encapsulated drug. The feasibility of this technique was demonstrated by Lamichhane and coworkers [15][14], who labeled the liposome’s surface with 111In and the carboplatin derivative it encapsulated with 18F. More attention has also been paid in recent times to the search for new radiotracers compatible with the half-life of liposomes, and 52Mn has been identified as a suitable radionuclide for this purpose [16][18]. Pharmacokinetic studies have revealed a marked variability in liposome uptake by different tumors. This may be linked to the tumor’s mass, as Lin and coworkers [17][8] found in their study; small tumors showed growth inhibition with all the treatment regimens tested (liposomes containing chemotherapeutics and/or radionuclides), whereas the growth of large tumors was only significantly inhibited by a combination of chemo- and radiotherapy. It is not unusual to see a different liposome uptake in different patients with the same tumor or different tumors in the same patient. Tumor deposition is due mainly to the EPR effect, which could complicate pretreatment planning and hamper predictions regarding a patient’s prognosis [18][12]. The abovementioned studies on the pharmacokinetic properties of radiolabeled liposomes enabled tumor deposition and distribution to be quantified [19][9], making it possible to identify patients mostly likely to respond to a liposomal therapy. Before testing liposomes in humans, it was important to demonstrate the feasibility of radiolabeling preformed liposomal formulations. This was the goal of a study by Edmonds and coworkers [18][12], who successfully labeled liposomal formulations of drugs containing metal-binding motifs (e.g., doxorubicin and alendronate) with PET isotopes (e.g., 89Zr, 52Mn, and 64Cu) using metal ionophores (e.g., hydroxyquinoline). The uptake of liposomal formulations can also be studied by recreating liposomes with the same lipid composition. This was achieved in vivo by Ito and coworkers [20][10], who synthesized liposomes with the same lipid composition as Doxil (a liposomal formulation of doxorubicin); they found a correlation between the therapeutic effect of Doxil and a histological factor associated with the EPR effect. Clinical studies on the biodistribution of liposomal formulations of chemotherapeutics in patients were made by Arietta and coworkers [21][22][19,20] and Lee and coworkers [23][21]. Arietta’s group examined the antitumor activity of a therapy combining liposomal doxorubicin (LD) with cisplatin in patients with malignant pleural mesothelioma. They labeled the LD with 99mTc and found that patients who showed a 99mTc-LD uptake of 75% or more had significantly better rates of response, progression-free survival, and overall survival than patients with uptake levels below 75%. The authors concluded that 99mTc-LD uptake could be an important biomarker for use in assessing the results of therapy with LD and cisplatin [21][22][19,20]. Lee’s group radiolabeled MM-302, an HER2-targeted Doxil formulation, with 64Cu. After promising preliminary in vitro results [19][9], the liposomal vesicles were administered in humans [23][21], and the 64Cu-MM-302 uptake was found to vary considerably, both across multiple lesions in the same patient and across different patients. A high uptake in the liver was due to the physiological metabolism of liposomes.3. Avidin–Biotin Interaction
Avidin is a 66 kDa highly glycosylated, positively charged protein (isoelectric point~10) derived from egg white. It is tetrameric, and each monomer has a strong affinity for biotin (Kd = 10−15) [24][25][26][28,30,38]. The strength of the avidin–biotin interaction is such that it is considered irreversible, and this explains why its applications have been the object of so much interest. For example, it has been studied in the sphere of tumor-targeted therapy for use in a pretargeting approach, which consists of delivering MoAbs and radionuclides separately. The radionuclide delivery is delayed until the MoAbs have reached the maximum tumor-to-normal tissue ratio [27][23], and the avidin–biotin interaction ensures the binding of the radiolabeled agent to the previously delivered antibody [28][22]. Two- or three-step protocols have been used in this setting (Figure 12).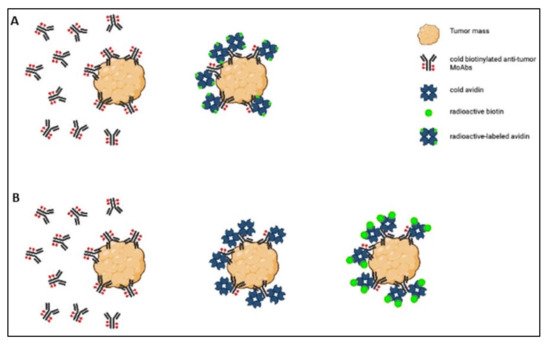
Figure 12. Scheme of the two-step (A) and three-step (B) protocols.
