Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Lindsay Dong and Version 2 by Lindsay Dong.
Stroke recovery processes include angiogenesis and neuroplasticity and advances in neuroimaging techniques may provide indirect description of this action and become quantifiable indicators of these processes as well as responses to the therapeutical interventions. This means that neuroimaging and neurophysiological methods can be used as biomarkers—to make a prognosis of the course of stroke recovery and define patients with great potential of improvement after treatment. This approach is most likely to lead to novel rehabilitation strategies based on categorizing individuals for personalized treatment.
- stroke
- recovery
- neurorehabilitation
- neuroimaging
- neuroplasticity
- angiogenesis
1. Introduction
Stroke is an acute cerebral, spinal, or retinal vascular accident with neurological dysfunction that persists longer than 24 h, or one of any duration when infarction or hemorrhage corresponding to symptoms is demonstrated by imaging (computed tomography/ magnetic resonance scans) or autopsy [1]. The clinical symptoms are very heterogenous and conditional on the topography of damage [2]. There is effective, specific treatment for acute ischemic stroke available, such as thrombolysis (intravenous administration of tissue plasminogen activator) or endovascular thrombectomy [3]. Furthermore, ischemic stroke patients should be administrated with antiplatelet or anticoagulant drugs (depending on the etiology of ischemia) as secondary stroke prevention [4]. The procedure for acute hemorrhage stroke is based on intensive blood pressure and intracranial pressure reduction. In addition, early open-surgery evacuation or drainage of hematoma might be beneficial and should be considered in specific cases [5]. Following the onset of stroke, natural, spontaneous processes of recovery occur, but are generally incomplete and difficult to predict amongst individuals. By fostering angiogenic and neuroplastic changes, restitution of function in damaged, nearby, or distant (but functionally or structurally connected) regions to the lesion may become more efficient [6]. Therefore, novel therapeutic approaches of stroke rehabilitation should be a focus of attention among researchers. Neurorehabilitation is intended to aid stroke patients to become as independent as possible and should be introduced soon after the patient’s condition stabilizes. Novel rehabilitation strategies are specific to the individual’s therapeutic goal, so directed at affected functional areas including motor impairments (most frequent), gait disturbance, speech disorders, cognitive failure, vision disturbances, etc. Therefore, it is appropriate to provide post-stroke rehabilitation by an interdisciplinary medical team together with physicians, psychiatrists or psychologists, neurologists, physical and occupational therapists, speech-language pathologists, nutritionists, and others [7]. Increasingly, novel technology applications are employed in post-stroke procedures, such as rehabilitation robots—as therapy devices (to train lost motor function) as well as assistive devices (to compensate lost skills) [8]. When considering the strategies of modern post-stroke rehabilitation, the importance of non-invasive neuromodulation techniques including repetitive transcranial magnetic stimulation and transcranial direct current stimulation should also be emphasized [9,10,11,12][9][10][11][12]. Furthermore, virtual reality programs on mobile devices may constitute a time-efficient, clinically effective, easy-to-implement and goal-oriented tool for upper extremity stroke rehabilitation [13].
For outcome prognosis, recovery processes such as angiogenesis and neuroplasticity should be analyzed. Angiogenesis is a renewed growth of blood vessels to restore blood supply to the damaged brain tissue and it occurs after stroke [17,18][14][15]. This restorative action is undoubtedly beneficial after stroke; it was proved that there is a stroke-related period of heightened vascular plasticity that is correlated with restoration of blood flow and then predictive of motor function recovery [19][16]. However, it is also worth remembering that angiogenic factors, such as VEGF and MMPs, contribute to blood brain barrier injury and may lead to oedema formation or haemorrhagic transformation [20][17]. Neuroplasticity definition contains adaptive structural and functional processes, including synaptogenesis, neurogenesis and neuroprotection. The critical period for post-stroke recovery and neuroplasticity is considered the acute (0–7 days) and the early subacute (7 days-3 months) phase [8]. In the acute phase, secondary neuronal networks are exploited to preserve function, whereas in the subacute stage, new synaptic connections are formed, and in the chronic phase, remodeling by axonal sprouting and then reorganization occurs [21][18]. There is also enhanced expression of growth associated genes and proteins observed [22][19], changes in GABA (gamma-aminobutyric acid), NMDA (N-methyl-D-aspartate) receptor subtypes and upregulation of NMDA receptors to increase brain excitability [23][20]. Diaschisis can be demonstrated by neuroimaging techniques that evaluate changes in cerebral blood flow, neurotransmitters, and metabolism action in regions distant from the lesion.
2. Neuroimaging Techniques Dedicated to Stroke
Neuroimaging is usually associated with differentiating ischemic from hemorrhage stroke in the acute phase; it also has a crucial role in ischemic stroke patients’ selection for novel treatment options, including late window thrombectomy.Figure 1 shows the emerging roles of stroke biomarkers [26][21]. Figure 2 presents a classification of neuroimaging techniques with potential for use in stroke recovery prognosis. Neuroimaging stroke recovery biomarkers include measures of structure and function [16][22]. To evaluate structure, people commonly use characteristics including infarct volume, extent of cortical or white matter injury, white matter integrity, and percentage of corticospinal tract injury. Functional assessment should be focused on features including activation within ipsilesional and contralesional sites, interhemispheric balance, resting state functional connectivity, task-based synchronization, and desynchronization, as well as cortical excitability, facilitation, and inhibition [27][23].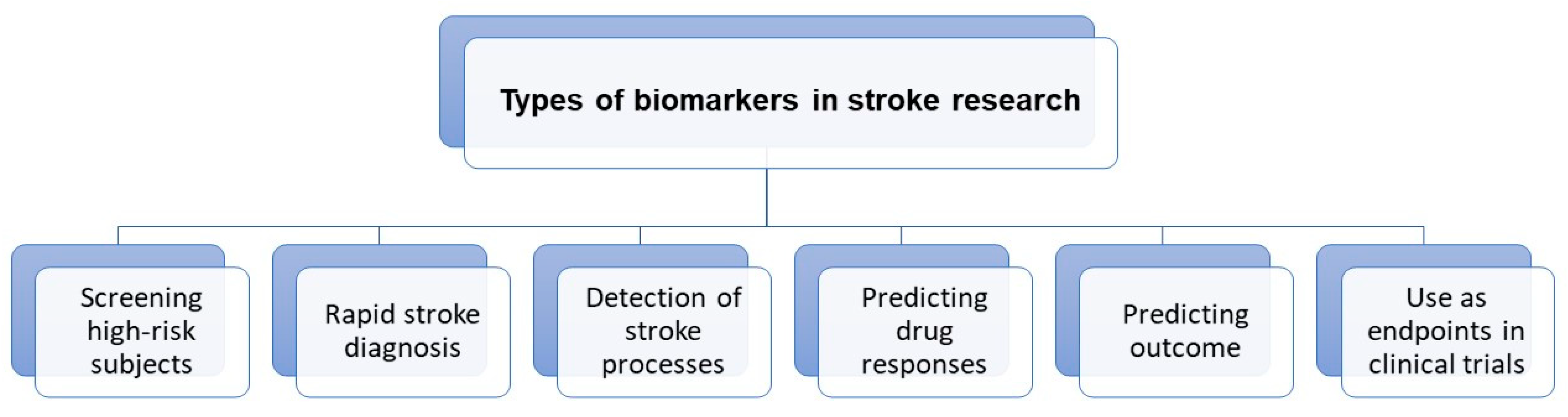
Figure 1. Roles of stroke biomarkers.
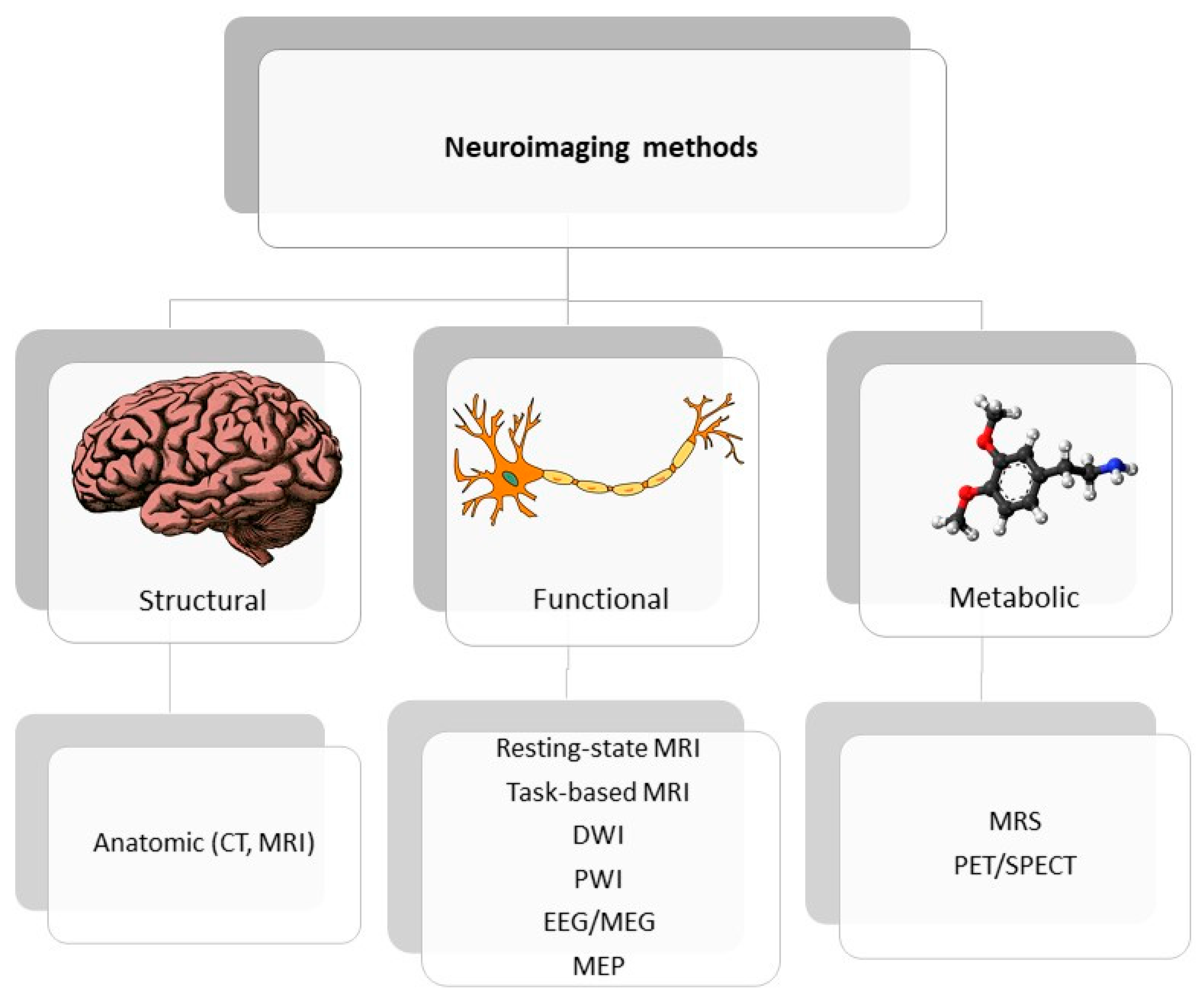
Figure 2. Classification of neuroimaging methods; CT—computer tomography, EEG—electroencephalography, MEP—motor evoked potential, MRI—magnetic resonance imaging, MRS—magnetic spectroscopy, PET/SPECT—positron emission tomography/ single photon emission computed tomography.
3. Stroke Recovery Prognosis Based on Selected Neuroimaging Measurements
3.1. Potential Angiogenic Biomarkers of Stroke Recovery
Angiogenesis after stroke is a complex and multistep process, involving (after gene transcription and releasing proangiogenic factors) endothelial cell proliferation, vascular sprouting, and finally microvessel formation [28][24]. In the current state of knowledge, imaging techniques allow evaluation of a wide spectrum of structural and functional tissue features. Recent developments in magnetic resonance imaging (MRI) techniques presented the possibility of assessing tissue perfusion and estimating the quantification of various features in the vascular network, including microvascular cerebral blood volume (CBV) or microvascular density [29][25]. In an experimental study of Yanev et al., steady-state contrast-enhanced (ssCE-) MRI with a long-circulating blood pool was applied to characterize the model of vascular reorganization within the ischemic lesion and in secondarily affected areas, from subacute to chronic stages, after focal cerebral stroke [30][26]. Results showed that dynamic revascularization in the perilesional area and progressive neovascularization in non-ischemic connected areas were found. These phenomena may contribute to non-neuronal tissue remodeling and be relevant in post-stroke recovery. In turn, the initial stage of angiogenesis might be observed by MRI techniques as blood-brain barrier leakage, because BBB permeability is associated with endothelial cell proliferation and vascular sprouting [31][27]. To quantify BBB integrity, the dynamic contrast-enhanced MRI (DCE-MRI) with gadolinium chelates could be included in patients with changes in MRI signals, which occur as a consequence of leakage of an intravenously injected contrast into the interstitial area [32][28]. According to Pradillo et al., DCE-MRI was shown to be a useful and non-invasive technique for evaluation of vascular function and angiogenesis processes [33][29]. The most prevalent investigation into direct assessment of vessels is angiography MR. However, the direct depiction of cerebrovascular remodeling or angiogenesis is very rare in clinical usage, because of the relatively low spatial resolution of MRI. Endothelial sprouting and microvessel formation proceed at length scales that are at minimum one order of magnitude lower than the current technology provides. Nevertheless, high-resolution T1-contrast based on ultra-short echo time MR angiography (UTE-MRA) was carried out to visualize macro- and microvasculature and their association with ischemic edema status in transient middle cerebral artery occlusion rat models [34][30]. Although the presented results are very promising, the limitations associated with MRI as a tool for demonstrating post-stroke angiogenesis should be taken into consideration. Elevation of CBV is not only specific for neovascularization but may also contribute to the collateral flow and vasodilatation of existing vessels. Likewise, contrast agent leakage may arise in relation to angiogenesis as well as a result of BBB disruption in response to vascular pathology and hemorrhage [31][27]. To ensure new insights into the role of vascular remodeling in functional recovery after stroke, more studies need to be performed. The use of modern technologies may be very valuable as a monitoring tool for possible future therapies designed to support neovascularization in post-stroke patients, thus providing the possibility of introducing personalized therapies.
3.2. Neuroplastic/Neurogenic Markers of Stroke Recovery
Understanding neural mechanisms of brain tissue damage as well as regeneration processes could be essential for predicting recovery and monitoring therapy. Such neural mechanisms of recovery involve in particular the perilesional tissue in the injured hemisphere, but also the contralateral hemisphere, subcortical and spinal regions [35][31]. All those processes that support recovery, termed neuroplasticity, are possible to identify as structural and functional brain changes in various neuroimaging techniques, such as magnetic resonance imaging (MRI), functional MRI, and functional magnetic spectroscopy (MRS) [36][32]. What is more, by using neurophysiological agents such as electroencephalography (EEG), people can map the brain activity and by using transcranial magnetic stimulation (TMS), it is also possible to test the influence of specific brain regions on motor learning and post-stroke recovery [37][33]. MRI is an essential clinical tool for diagnosing stroke severity, implementing treatment and predicting outcome [38][34]. Multimodal MRI reveals various parameters that help determine stroke mechanisms which affect recovery, such as differentiation of ischemic core from ischemic penumbra. The ischemic core is an area of infarction, which develops rapidly after artery occlusion. The differentiating parameter is the cBV, which is kept in the penumbra zone and decreased in the ischemic core. Using MRI technology, ischemic lesions can be identified with high precision, using diffusion-weighted image (DWI). Perfusion-weighted image (PWI) in turn can identify ischemic penumbral tissue [39][35]. To assign areas with PWI-DWI mismatch (the area difference when the perfusion lesion is larger than the diffusion lesion) is to identify representative salvageable tissue that may be responsible in recovery [40][36]. There is agreement as to the usefulness of characterizing the ischemic penumbra at the acute stage in relation to predicting motor outcomes. However, there are also data which suggest that the location of ischemic penumbra, instead of volume, could predict outcome and affect motor recovery [41][37]. MRI also delivers a method for assessing indices of white matter integrity and remodeling following ischemic stroke through diffusion-based methods. Measures of corticospinal tract (CST) white matter integrity is possible by diffusion tensor imaging (DTI) that uses anisotropic diffusion to estimate the axonal (white matter) organization of the brain. Ratio and asymmetry index of fractional anisotropy (FA) between ipsi- and contralesional corticospinal tracts (CSTs) is a very popular predictor in DTI studies. In general, a lower FA value of the ipsilesional CST may indicate greater damage tp the CST that can lead to more Wallerian degeneration of CST axons [42][38]. It has been proved that corticospinal tract injury is a valuable predictor of motor recovery in acute and post-acute stages [43,44,45][39][40][41]. In turn, Doughty et al. discovered that FA reduction of the CST (detected in the acute phase of stroke) present fractional predictive value to motor outcomes at 3 months [46][42]. Essentially, FA value can also be influenced by other factors (not only damage of CST), such as white matter architecture, so it should be carefully considered as a biomarker of brain impairment and poor recovery. After stroke damage, people can observe a dynamic process of changing brain activation patterns. Measurement of brain function presents complexities that do not increase with the measurement of anatomy. The way to follow this complexity is functional MRI (fMRI), which measures brain activity by detecting changes associated with blood flow. It is considered that neuronal activation and cerebral blood flows are coupled [53][43]. The most common form of fMRI is based on the blood-oxygen-level dependent (BOLD) contrast, which can indirectly measure neural activity based on changes in blood flow and deoxyhemoglobin concentration [54][44]. To activate the brain with fMRI, a specific behavioral paradigm must be executed by a patient; it should be performed on command, correctly and on time. Therefore, the behavioral paradigm should also be carefully selected to investigate the brain’s functional field of interest. However, post-stroke motor impairments can make even simple motor performance difficult; thus, resting-state imaging is an attractive method for studying stroke network activity. With this technique, the functional connectivity represents the synchrony of intrinsic blood oxygen level-dependent (BOLD) signal fluctuations among different brain regions [55][45]. Functional connectivity that reflects the integrity of various motor and non-motor networks is associated with stroke outcome [56,57][46][47]. The next technique that allows assessment of the function of brain tissue is MRS (magnetic resonance spectroscopy). The presence and concentration of various metabolites is analyzed based on the principle that the distribution of electrons within an atom cause nuclei in different molecules to experience a slightly different magnetic field. In a study by Blicher et al., the higher GABA level in ipsilesional M1 was related to better motor function improvements after constraint-induced therapy [62][48]. Electroencephalography (EEG) is the most common, non-invasive method to record spontaneous or evoked electrical oscillation at various frequencies of the brain and, importantly, is one of the few mobile techniques available, unlike CT and MRI. Assessment of neuronal oscillations with electro or magneto-encephalography may supply an easy, available method to evaluate the balance between excitatory and inhibitory cortical actions [64][49]. EEG signals can identify sensitive changes in brain activity that cannot be detected by clinical measures. Furthermore, quantification of the EEG signal before and after treatment (rehabilitation) may evaluate neuroplasticity near the lesion and within whole-brain networks. The general findings, suggesting bad recovery in post-stroke patients investigated with EEG or MEG at the acute or subacute stages, indicate predominant inhibitory processes in the perilesional areas of cortex, shown by increased low-frequency oscillations [65,66][50][51].References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089.
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654.
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418.
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467.
- De Oliveira Manoel, A.L. Surgery for spontaneous intracerebral hemorrhage. Crit. Care 2020, 24, 45.
- Dancause, N.; Barbay, S.; Frost, S.B.; Plautz, E.J.; Chen, D.; Zoubina, E.V.; Stowe, A.M.; Nudo, R.J. Extensive cortical rewiring after brain injury. J. Neurosci. 2005, 25, 10167–10179.
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169.
- Klamroth-Marganska, V. Stroke Rehabilitation: Therapy Robots and Assistive Devices. Adv. Exp. Med. Biol. 2018, 1065, 579–587.
- Chieffo, R.; Comi, G.; Leocani, L. Noninvasive Neuromodulation in Poststroke Gait Disorders: Rationale, Feasibility, and State of the Art. Neurorehabil. Neural Repair 2016, 30, 71–82.
- Breining, B.L.; Sebastian, R. Neuromodulation in Post-stroke Aphasia Treatment. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 44–56.
- Beuter, A.; Balossier, A.; Vassal, F.; Hemm, S.; Volpert, V. Cortical stimulation in aphasia following ischemic stroke: Toward model-guided electrical neuromodulation. Biol. Cybern. 2020, 114, 5–21.
- Powell, K.; White, T.G.; Nash, C.; Rebeiz, T.; Woo, H.H.; Narayan, R.K.; Li, C. The Potential Role of Neuromodulation in Subarachnoid Hemorrhage. Neuromodulation 2022.
- Choi, Y.H.; Paik, N.J. Mobile Game-based Virtual Reality Program for Upper Extremity Stroke Rehabilitation. J. Vis. Exp. 2018, 133, 56241.
- Arai, K.; Jin, G.; Navaratna, D.; Lo, E.H. Brain angiogenesis in developmental and pathological processes: Neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009, 276, 4644–4652.
- Yanev, P.; Dijkhuizen, R.M. In vivo imaging of neurovascular remodeling after stroke. Stroke 2012, 43, 3436–3441.
- Williamson, M.R.; Franzen, R.L.; Fuertes, C.J.A.; Dunn, A.K.; Drew, M.R.; Jones, T.A. A Window of Vascular Plasticity Coupled to Behavioral Recovery after Stroke. J. Neurosci. 2020, 40, 7651–7667.
- Adamczak, J.; Hoehn, M. Poststroke angiogenesis, con: Dark side of angiogenesis. Stroke 2015, 46, e103–e104.
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17.
- Li, S.; Carmichael, S.T. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol. Dis. 2006, 23, 362–373.
- Redecker, C.; Wang, W.; Fritschy, J.M.; Witte, O.W. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: Mediation by NMDA-dependent processes. J. Cereb. Blood Flow Metab. 2002, 22, 1463–1475.
- Bang, O.Y. Advances in biomarker for stroke patients: From marker to regulator. Precis. Future Med. 2017, 1, 32–42.
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Leff, A.P.; Copland, D.A.; Carey, L.M.; et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 480–493.
- Jones, T.A.; Kleim, J.A.; Greenough, W.T. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: A quantitative electron microscopic examination. Brain Res. 1996, 733, 142–148.
- Zhu, H.; Zhang, Y.; Zhong, Y.; Ye, Y.; Hu, X.; Gu, L.; Xiong, X. Inflammation-Mediated Angiogenesis in Ischemic Stroke. Front. Cell. Neurosci. 2021, 15, 652647.
- Callewaert, B.; Jones, E.A.V.; Himmelreich, U.; Gsell, W. Non-Invasive Evaluation of Cerebral Microvasculature Using Pre-Clinical MRI: Principles, Advantages and Limitations. Diagnostics 2021, 11, 926.
- Yanev, P.; Seevinck, P.R.; Rudrapatna, U.S.; Bouts, M.J.; van der Toorn, A.; Gertz, K.; Kronenberg, G.; Endres, M.; van Tilborg, G.A.; Dijkhuizen, R.M. Magnetic resonance imaging of local and remote vascular remodelling after experimental stroke. J. Cereb. Blood Flow Metab. 2017, 37, 2768–2779.
- Yang, Y.; Torbey, M.T. Angiogenesis and Blood-Brain Barrier Permeability in Vascular Remodeling after Stroke. Curr. Neuropharmacol. 2020, 18, 1250–1265.
- Merali, Z.; Huang, K.; Mikulis, D.; Silver, F.; Kassner, A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS ONE 2017, 12, e0171558.
- Pradillo, J.M.; Hernández-Jiménez, M.; Fernández-Valle, M.E.; Medina, V.; Ortuño, J.E.; Allan, S.M.; Proctor, S.D.; Garcia-Segura, J.M.; Ledesma-Carbayo, M.J.; Santos, A.; et al. Influence of metabolic syndrome on post-stroke outcome, angiogenesis and vascular function in old rats determined by dynamic contrast enhanced MRI. J. Cereb. Blood Flow Metab. 2020, 41, 1692–1706.
- Kang, M.; Jin, S.; Lee, D.; Cho, H. MRI Visualization of Whole Brain Macro- and Microvascular Remodeling in a Rat Model of Ischemic Stroke: A Pilot Study. Sci. Rep. 2020, 10, 4989.
- Alia, C.; Spalletti, C.; Lai, S.; Panarese, A.; Lamola, G.; Bertolucci, F.; Vallone, F.; Di Garbo, A.; Chisari, C.; Micera, S.; et al. Neuroplastic Changes Following Brain Ischemia and their Contribution to Stroke Recovery: Novel Approaches in Neurorehabilitation. Front. Cell. Neurosci. 2017, 11, 76.
- Auriat, A.M.; Neva, J.L.; Peters, S.; Ferris, J.K.; Boyd, L.A. A Review of Transcranial Magnetic Stimulation and Multimodal Neuroimaging to Characterize Post-Stroke Neuroplasticity. Front. Neurol. 2015, 6, 226.
- Rolle, C.E.; Baumer, F.M.; Jordan, J.T.; Berry, K.; Garcia, M.; Monusko, K.; Trivedi, H.; Wu, W.; Toll, R.; Buckwalter, M.S.; et al. Mapping causal circuit dynamics in stroke using simultaneous electroencephalography and transcranial magnetic stimulation. BMC Neurol. 2021, 21, 280.
- Pinto, A.; Mckinley, R.; Alves, V.; Wiest, R.; Silva, C.A.; Reyes, M. Stroke Lesion Outcome Prediction Based on MRI Imaging Combined With Clinical Information. Front. Neurol. 2018, 9, 1060.
- González, R.G. Clinical MRI of acute ischemic stroke. J. Magn. Reson. Imaging 2012, 36, 259–271.
- Bracard, S. Predicting Tissue Viability in Ischemic Stroke with Diffusion and Perfusion MRI. Open Access J. Neurol. Neurosurg. 2019, 10, 555799.
- Rosso, C.; Samson, Y. The ischemic penumbra: The location rather than the volume of recovery determines outcome. Curr. Opin. Neurol. 2014, 27, 35–41.
- Puig, J.; Pedraza, S.; Blasco, G.; Daunis-I-Estadella, J.; Prats, A.; Prados, F.; Boada, I.; Castellanos, M.; Sánchez-González, J.; Remollo, S.; et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. Am. J. Neuroradiol. 2010, 31, 1324–1330.
- Bigourdan, A.; Munsch, F.; Coupé, P.; Guttmann, C.R.; Sagnier, S.; Renou, P.; Debruxelles, S.; Poli, M.; Dousset, V.; Sibon, I.; et al. Early Fiber Number Ratio Is a Surrogate of Corticospinal Tract Integrity and Predicts Motor Recovery After Stroke. Stroke 2016, 47, 1053–1059.
- Lin, D.J.; Cloutier, A.M.; Erler, K.S.; Cassidy, J.M.; Snider, S.B.; Ranford, J.; Parlman, K.; Giatsidis, F.; Burke, J.F.; Schwamm, L.H.; et al. Corticospinal Tract Injury Estimated From Acute Stroke Imaging Predicts Upper Extremity Motor Recovery After Stroke. Stroke 2019, 50, 3569–3577.
- Wen, H.; Alshikho, M.J.; Wang, Y.; Luo, X.; Zafonte, R.; Herbert, M.R.; Wang, Q.M. Correlation of Fractional Anisotropy With Motor Recovery in Patients With Stroke After Postacute Rehabilitation. Arch. Phys. Med. Rehabil. 2016, 97, 1487–1495.
- Doughty, C.; Wang, J.; Feng, W.; Hackney, D.; Pani, E.; Schlaug, G. Detection and Predictive Value of Fractional Anisotropy Changes of the Corticospinal Tract in the Acute Phase of a Stroke. Stroke 2016, 47, 1520–1526.
- Zilles, K.; Amunts, K. Anatomical Basis for Functional Specialization. In fMRI: From Nuclear Spins to Brain Functions; Uludag, K., Ugurbil, K., Berliner, L., Eds.; Springer: Boston, MA, USA, 2015; pp. 27–66.
- Crofts, A.; Kelly, M.E.; Gibson, C.L. Imaging Functional Recovery Following Ischemic Stroke: Clinical and Preclinical fMRI Studies. J. Neuroimaging 2020, 30, 5–14.
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139.
- Grefkes, C.; Fink, G.R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014, 13, 206–216.
- Almeida, S.R.; Vicentini, J.; Bonilha, L.; De Campos, B.M.; Casseb, R.F.; Min, L.L. Brain Connectivity and Functional Recovery in Patients With Ischemic Stroke. J. Neuroimaging 2017, 27, 65–70.
- Blicher, J.U.; Near, J.; Næss-Schmidt, E.; Stagg, C.J.; Johansen-Berg, H.; Nielsen, J.F.; Østergaard, L.; Ho, Y.C. GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil. Neural Repair 2015, 29, 278–286.
- Rabiller, G.; He, J.-W.; Nishijima, Y.; Wong, A.; Liu, J. Perturbation of Brain Oscillations after Ischemic Stroke: A Potential Biomarker for Post-Stroke Function and Therapy. Int. J. Mol. Sci. 2015, 16, 5605.
- Zappasodi, F.; Pasqualetti, P.; Rossini, P.M.; Tecchio, F. Acute Phase Neuronal Activity for the Prognosis of Stroke Recovery. Neural Plast. 2019, 2019, 1971875.
- Laaksonen, K.; Helle, L.; Parkkonen, L.; Kirveskari, E.; Mäkelä, J.P.; Mustanoja, S.; Tatlisumak, T.; Kaste, M.; Forss, N. Alterations in spontaneous brain oscillations during stroke recovery. PLoS ONE 2013, 8, e61146.
More
