Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Conner Chen and Version 3 by Conner Chen.
Iron is a vital nutrient, the deficiency of which is responsible for several symptoms of anemia. Iron is responsible for the regulation of several cell functions.
- iron
- oxidative stress
- redox-active iron
- non-transferrin bound iron
1. Introduction
Iron is a vital nutrient, the deficiency of which is responsible for several symptoms of anemia. Many people suffer from iron deficiency, especially children and young women [1]. Iron overload is responsible for hereditary as well as secondary hemochromatoses [2][3][4][5]. Even under normal conditions, iron may cause pathological damage depending on local conditions to produce redox-active iron. For example, free catalytic iron may be present in ischemic [6][7] or inflammatory lesions [8][9] and thus result in iron-induced oxidative stress because the pH in such lesions decreases [10], owing to which iron is solubilized as ions. Therefore, iron is the mastermind behind many systemic and local human diseases. Iron acts as a double-edged sword [11] and has to be strictly controlled and sequestered so as to not generate reactive oxygen species (ROS).
2. Basic Properties of Iron
Iron is a 3d transition element containing five incompletely occupied d orbitals and has an electron configuration of [Ar]3d64s2. In the human body, iron is usually present in two stable ionic states—ferric or ferrous.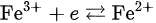 Ferric ions have an electron configuration of [Ar]3d5, with a 6S5/2 spectral term in the ground state as well as a completely symmetric structure. At its physiological pH 7, ferric ion is substantially insoluble and is deposited as ferric hydroxide colloids [12][13]. It is recommended that ferric ions be stored in strong acids, such as nitrate, in the laboratory. Researchers have often experienced that iron is deposited on the walls of test tubes containing an aged solution even if it is stored with some chelating ligand. To exist in the ionic state in human fluids, ferric ions need a chelating ligand. When ligands in a ferric ion coordinate, five symmetric d-orbitals split the ground energy into two energy levels–t2g and eg–in a typical octahedral geometry. The energy difference Δ between t2g and eg is known as the crystal field splitting energy. Five electrons occupy each different orbital with the same upper spin in high-spin iron compounds, or the electrons occupy lower energy levels with electron pairs and only one electron has a half spin in the low-spin state. Iron bound to transferrin and many weak chelating ligands exist in ferric high-spin states because of the small Δ.
The solubility product of ferric ion is 10−39 M and [Fe3+] is 10−18 M at pH 7 [13]. According to the theory of hard and soft acids and bases, ferric ion is a hard acid that binds some hard bases, for example H2O and OH−. Ferric ion is insoluble in water and can form diiron, triiron, and polyiron coordination compounds. Not enough data on the reactivity of polyiron complexes in biology and medicine are available. Okazaki et al. [14] and Mizuno et al. [15] reported the role of diiron complexes in Fe-nta-induced renal injury and carcinogenesis. Fe-nta has a μ-oxo, μ-carbonato diiron complex [16] and it is suggested that the peroxide adduct of Fe-nta has a unique reactivity [17][18].
High-valent states of iron–Fe(IV), Fe(V), and Fe(VI)–have been reported in iron-containing enzymes and the model compounds. For example, iron may exist in the Fe(IV) transient state [19] in peroxidase reactions.
Ferrous ions have an [Ar]3d6 electron configuration, with a 5D4 spectral term, and typically possess tetrahedral or octahedral structures owing to ligand binding. Ferrous ions are much more soluble in water than ferric ions; however, they also easily become oxidized to ferric ions and deposit as iron colloids.
Ferric ions have an electron configuration of [Ar]3d5, with a 6S5/2 spectral term in the ground state as well as a completely symmetric structure. At its physiological pH 7, ferric ion is substantially insoluble and is deposited as ferric hydroxide colloids [12][13]. It is recommended that ferric ions be stored in strong acids, such as nitrate, in the laboratory. Researchers have often experienced that iron is deposited on the walls of test tubes containing an aged solution even if it is stored with some chelating ligand. To exist in the ionic state in human fluids, ferric ions need a chelating ligand. When ligands in a ferric ion coordinate, five symmetric d-orbitals split the ground energy into two energy levels–t2g and eg–in a typical octahedral geometry. The energy difference Δ between t2g and eg is known as the crystal field splitting energy. Five electrons occupy each different orbital with the same upper spin in high-spin iron compounds, or the electrons occupy lower energy levels with electron pairs and only one electron has a half spin in the low-spin state. Iron bound to transferrin and many weak chelating ligands exist in ferric high-spin states because of the small Δ.
The solubility product of ferric ion is 10−39 M and [Fe3+] is 10−18 M at pH 7 [13]. According to the theory of hard and soft acids and bases, ferric ion is a hard acid that binds some hard bases, for example H2O and OH−. Ferric ion is insoluble in water and can form diiron, triiron, and polyiron coordination compounds. Not enough data on the reactivity of polyiron complexes in biology and medicine are available. Okazaki et al. [14] and Mizuno et al. [15] reported the role of diiron complexes in Fe-nta-induced renal injury and carcinogenesis. Fe-nta has a μ-oxo, μ-carbonato diiron complex [16] and it is suggested that the peroxide adduct of Fe-nta has a unique reactivity [17][18].
High-valent states of iron–Fe(IV), Fe(V), and Fe(VI)–have been reported in iron-containing enzymes and the model compounds. For example, iron may exist in the Fe(IV) transient state [19] in peroxidase reactions.
Ferrous ions have an [Ar]3d6 electron configuration, with a 5D4 spectral term, and typically possess tetrahedral or octahedral structures owing to ligand binding. Ferrous ions are much more soluble in water than ferric ions; however, they also easily become oxidized to ferric ions and deposit as iron colloids.
Fe2+ + O2 → Fe2+ − O2 ⇄ Fe3+ − O2−· + Fe2+ → Fe3+ − O2 − Fe3+ → colloids
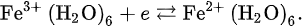
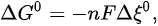
where n is the number of electrons and F is the Faraday constant. In the actual reaction, the change in Gibbs energy is as follows:
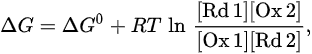
Table 1. Standard reduction potentials for some iron coordinate compounds. (1) ref [20]; (2) ref [21]; (3) ref [22].
| Redox Couple | Reduction Potential |
|---|---|
| Fe3+/Fe2+ | 0.771 (1) |
| Fe(OH)3/Fe(OH)2 | −0.56 (1) |
| [Fe(bipy)3+3]/[Fe(bipy)2+3] | 1.03 (1) |
| [Fe(phen)3+3]/[Fe(phen)2+3] | 1.147 (1) |
| Fe3+-nta/Fe2+-nta | 0.59 (2) |
| Fe3+-dtpa/Fe2+-dtpa | 0.03 (3) |
| Fe3+-edta/Fe2+-edta | 0.12 (3) |
| Fe3+-ADP/Fe2+-ADP | 0.10 (3) |
| Fe3+-DFO/Fe2+-DFO | −0.45 (3) |
| Fe3+-Tf/Fe2+-Tf | −0.40 (3) |
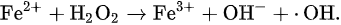
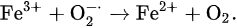
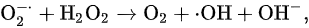 which is well known as the Haber–Weiss reaction [23]; however, Equation (3) cannot proceed without a catalytic iron.
A solution of ferrous irons and hydrogen peroxide has been used as the Fenton reagent to oxidize organic molecules. However, the actual mechanism underlying the Fenton reaction remains controversial [24]; it is unknown whether free hydroxy radicals are generated or if a coordination complex of iron and hydrogen peroxide [for example, Fe(IV)=O] attack organic molecules [25]. It is also possible that iron binds weakly to an organic molecule, after which hydrogen peroxide reacts at the site. The detailed mechanism may depend on the nature of the oxidized molecules and the reaction environments.
In the fields of biology and medicine, the spin trapping method has often been used to identify ROS in concerned systems including experiments on iron-induced oxidative stress. 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) has been frequently used as a spin trap because the spin adducts to superoxide and hydroxy radicals, DMPO-OOH and DMPO-OH (Figure 1), can be identified at a glance. A simulation of the DMPO spin adducts was performed using EasySpin software [26]. Spin trapping studies using DMPO have been performed to identify oxygen-derived free radicals in iron-induced oxidative stress, and DOMP-OH adducts have been reported as a direct evidence of the presence of hydroxy radical [27]. However, one must be cautious of DMPO spin trapping in iron-rich systems [28][29][30]. DMPO can chelate ferric ions, and non-specific DMPO adducts can be generated in iron-containing systems.
which is well known as the Haber–Weiss reaction [23]; however, Equation (3) cannot proceed without a catalytic iron.
A solution of ferrous irons and hydrogen peroxide has been used as the Fenton reagent to oxidize organic molecules. However, the actual mechanism underlying the Fenton reaction remains controversial [24]; it is unknown whether free hydroxy radicals are generated or if a coordination complex of iron and hydrogen peroxide [for example, Fe(IV)=O] attack organic molecules [25]. It is also possible that iron binds weakly to an organic molecule, after which hydrogen peroxide reacts at the site. The detailed mechanism may depend on the nature of the oxidized molecules and the reaction environments.
In the fields of biology and medicine, the spin trapping method has often been used to identify ROS in concerned systems including experiments on iron-induced oxidative stress. 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) has been frequently used as a spin trap because the spin adducts to superoxide and hydroxy radicals, DMPO-OOH and DMPO-OH (Figure 1), can be identified at a glance. A simulation of the DMPO spin adducts was performed using EasySpin software [26]. Spin trapping studies using DMPO have been performed to identify oxygen-derived free radicals in iron-induced oxidative stress, and DOMP-OH adducts have been reported as a direct evidence of the presence of hydroxy radical [27]. However, one must be cautious of DMPO spin trapping in iron-rich systems [28][29][30]. DMPO can chelate ferric ions, and non-specific DMPO adducts can be generated in iron-containing systems.
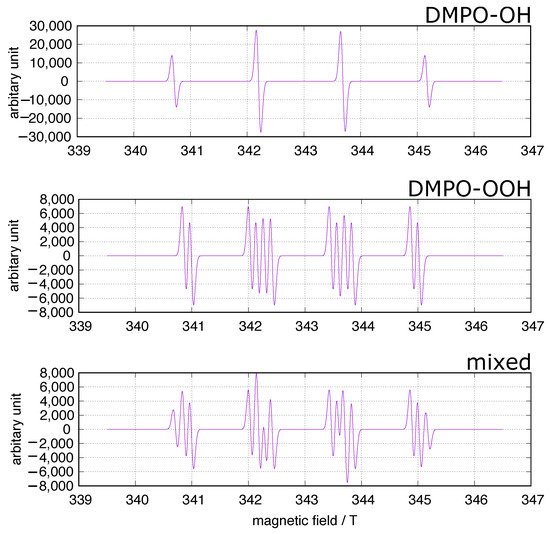
Figure 1. DMPO spin trapping. The simulation was performed using EasySpin software [26]. upper: DMPO-OH, hyperfine splitting constants (mT): AN = 1.49, AH = 1.48; middle: DMPO-OOH, hyperfine
References
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248.
- Crichton, R.R. Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, 4th ed.; Wiley: Hoboken, NJ, USA, 2016.
- Barton, J.C.; Acton, R.T. Hemochromatosis. J. Diabetes Res. 2017, 2017, 9826930.
- Kawabata, H. The mechanisms of systemic iron homeostasis and etiology, diagnosis, and treatment of hereditary hemochromatosis. Int. J. Hematol. 2018, 107, 31–43.
- Brissot, P.; Brissot, E. What’s Important and New in Hemochromatosis? Clin. Hematol. Int. 2020, 2, 143–148.
- Yamada, N.; Karasawa, T.; Wakiya, T.; Sadatomo, A.; Ito, H.; Kamata, R.; Watanabe, S.; Komada, T.; Kimura, H.; Sanada, Y.; et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: Potential role of ferroptosis. Am. J. Transpl. 2020, 20, 1606–1618.
- Blomgren, K.; Hagberg, H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic. Biol. Med. 2006, 40, 388–397.
- Lee, N.J.; Ha, S.K.; Sati, P.; Absinta, M.; Nair, G.; Luciano, N.J.; Leibovitch, E.C.; Yen, C.C.; Rouault, T.A.; Silva, A.C.; et al. Potential role of iron in repair of inflammatory demyelinating lesions. J. Clin. Investig. 2019, 129, 4365–4376.
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229.
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease; Elsevier: Amsterdam, The Netherlands, 2020.
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys Acta Mol. Cell Res. 2019, 1866, 118535.
- Jacob, A.; Worwood, M. Iron in Biochemistry and Medicine, II, 2nd ed.; Academic Press: New York, NY, USA, 1980.
- Crichton, R.R. Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012.
- Okazaki, Y. The Role of Ferric Nitrilotriacetate in Renal Carcinogenesis and Cell Death: From Animal Models to Clinical Implications. Cancers 2022, 14, 1495.
- Mizuno, R.; Kawabata, T.; Sutoh, Y.; Nishida, Y.; Okada, S. Oxidative renal tubular injuries induced by aminocarboxylate-type iron (III) coordination compounds as candidate renal carcinogens. Biometals 2006, 19, 675–683.
- Fujita, T.; Ohba, S.; Nishida, Y.; Goto, A.; Tokii, T. Tetrapotassium μ-carbonato-μ-oxo-bis dimethanol dihydrate. Acta Cryst. 1994, 50, 544–546.
- Nishida, Y.; Ito, Y.; Satoh, T. Origin of renal proximal tubular injuries by Fe(III)-nta chelate. Z Naturforsch. C J. Biosci. 2007, 62, 608–612.
- Nishida, Y.; Ito, S. Comparison on reactivity of Fe(III) and Al(III) compounds in the presence of hydrogen peroxide: Its relevance to possible origin for central nervous system toxicity by aluminum ion. Z Naturforsch. C J. Biosci. 1995, 50, 571–577.
- Hrycay, E.G.; Bandiera, S.M. The monooxygenase, peroxidase, and peroxygenase properties of cytochrome P450. Arch Biochem. Biophys. 2012, 522, 71–89.
- Rumble, J. CRC Handbook of Chemistry and Physics, 100th ed.; CRC Press: New York, NY, USA, 2019.
- Wang, Z.; Liu, C.; Wang, X.; Marshall, M.J.; Zachara, J.M.; Rosso, K.M.; Dupuis, M.; Fredrickson, J.K.; Heald, S.; Shi, L. Kinetics of reduction of Fe(III) complexes by outer membrane cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2008, 74, 6746–6755.
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2014.
- Filipovic, M.R.; Koppenol, W.H. The Haber-Weiss reaction-The latest revival. Free Radic. Biol. Med. 2019, 145, 221–222.
- Stanbury, D.M. The principle of detailed balancing, the iron-catalyzed disproportionation of hydrogen peroxide, and the Fenton reaction. Dalton Trans. 2022, 51, 2135–2157.
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10.
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55.
- Ranguelova, K.; Mason, R.P. The fidelity of spin trapping with DMPO in biological systems. Magn. Reson. Chem. 2011, 49, 152–158.
- Makino, K.; Hagiwara, T.; Hagi, A.; Nishi, M.; Murakami, A. Cautionary note for DMPO spin trapping in the presence of iron ion. Biochem. Biophys. Res. Commun. 1990, 172, 1073–1080.
- Makino, K.; Hagiwara, T.; Imaishi, H.; Nishi, M.; Fujii, S.; Ohya, H.; Murakami, A. DMPO spin trapping in the presence of Fe ion. Free Radic. Res. Commun. 1990, 9, 233–240.
- Makino, K.; Imaishi, H.; Morinishi, S.; Hagiwara, T.; Takeuchi, T.; Murakami, A.; Nishi, M. An artifact in the ESR spectrum obtained by spin trapping with DMPO. Free Radic. Res. Commun. 1989, 6, 19–28.
More
