Periodontal diseases have a wide range of pathophysiology.
Osteocytes make about 90% of the bone cell population and serve as the major cells for bone production, mineralization, and cell signaling regulation.
2. Hormones
Hormones are essentially characterized as a stimulants, inhibitors, or chemical messengers that, after being released into the systemic circulation, cause a specific alteration in the cellular activity of target sites.
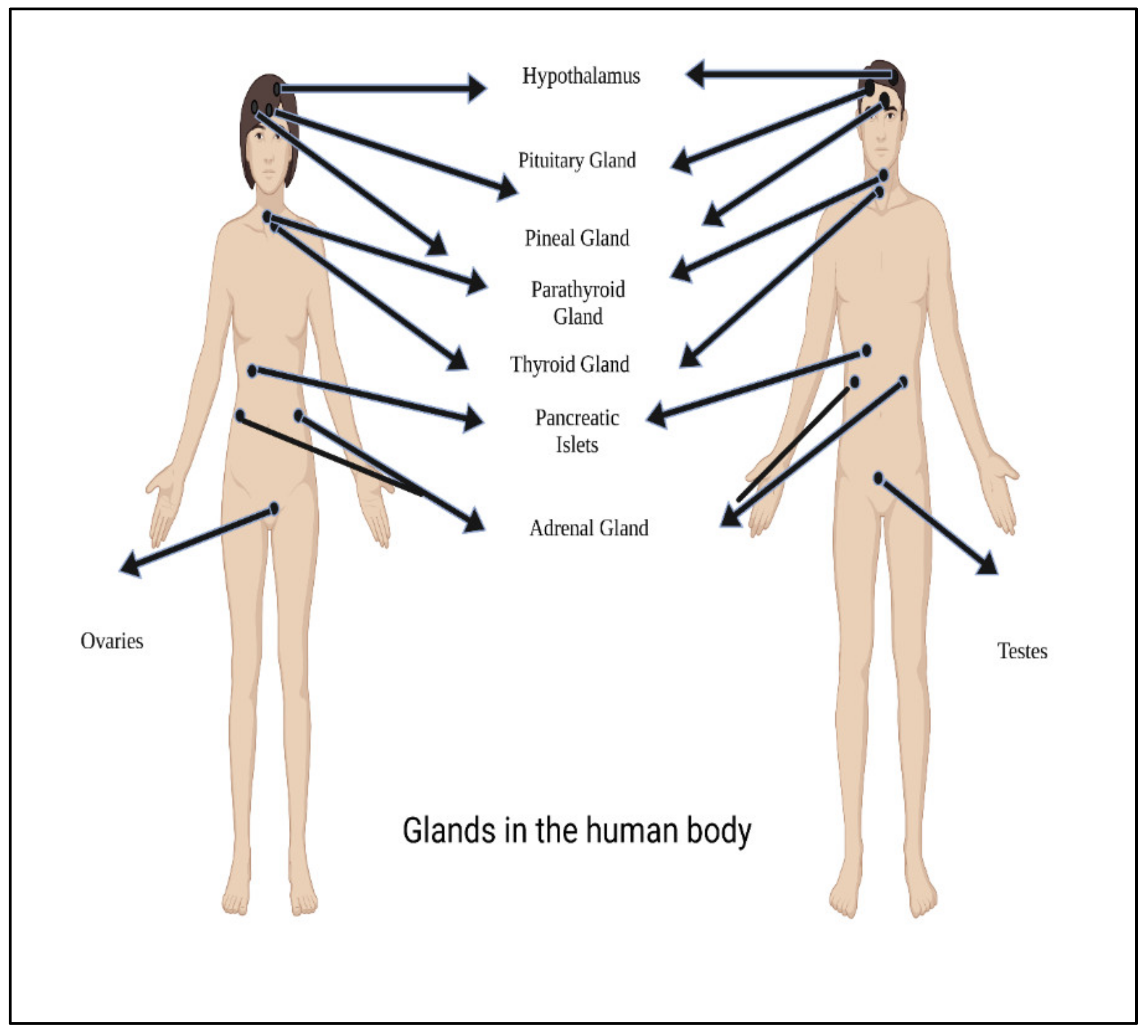
Figure 23 shows main glands in the human body.
Figure 23. Main glands in the human body.
Hormones are classified according to their composition, such as amino acids, tyrosine (catechol amines and thyroid hormones), tryptophan (serotonin), etc., as shown in
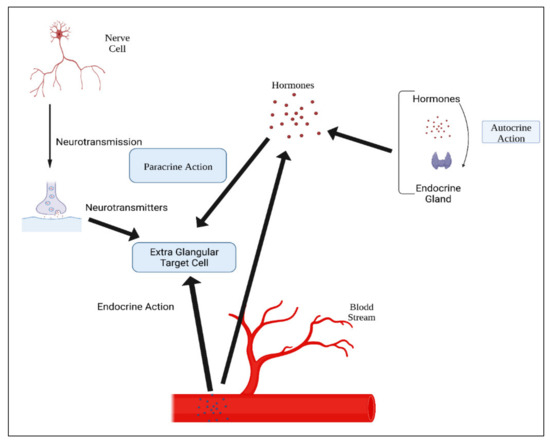
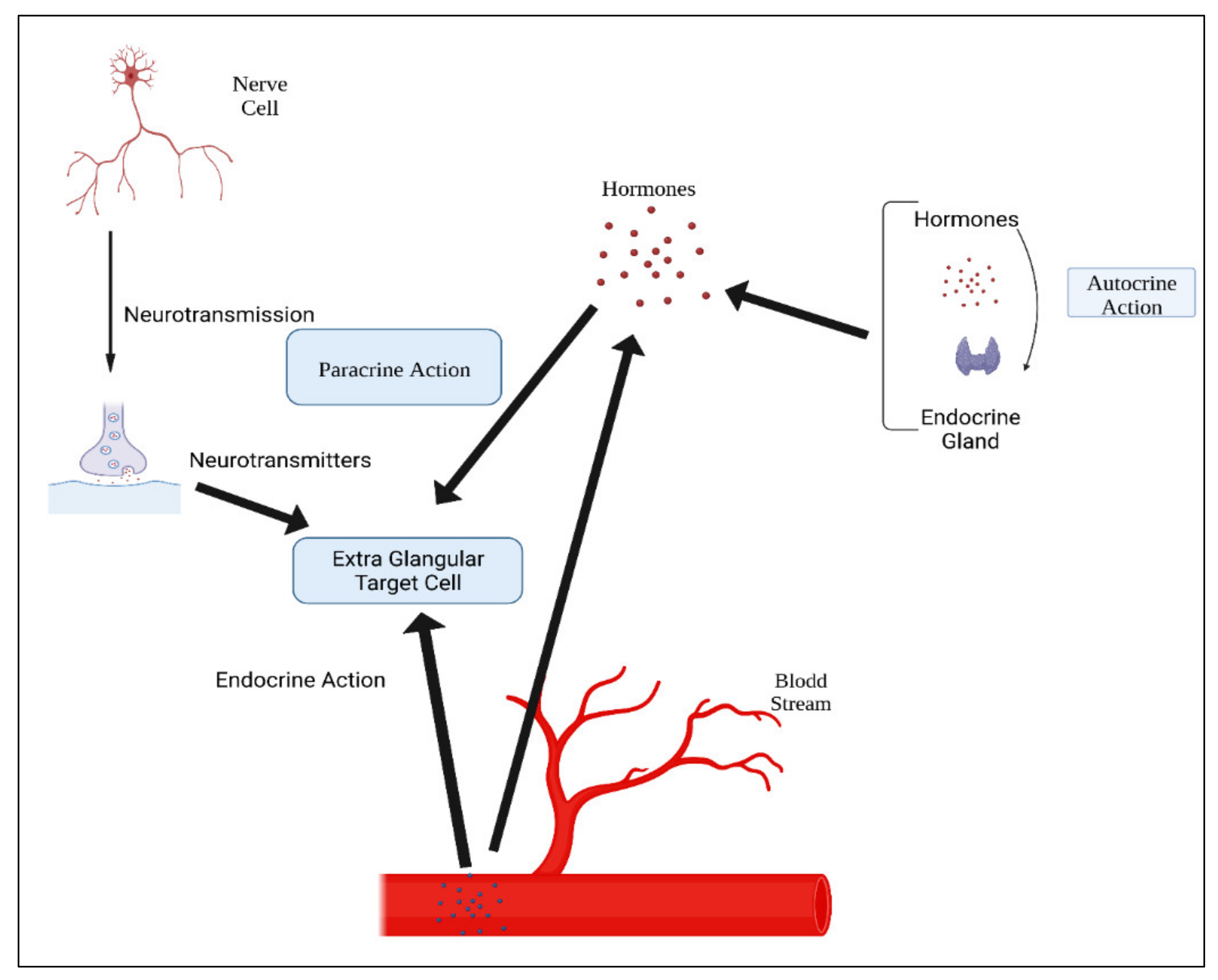
Table 13. Hormone action could be endocrine (site of their actions distant from the site of release), and may also be paracrine (functioning on nearby cells by diffusion), autocrine (acting on the secreting cells by diffusion), or intracrine (working in secreting cells without release). Agents that work in this manner are frequently referred to as factors instead of hormones, as shown in
Figure 34. Indeed, these substances (for example, hormones) may be generated in the majority of cells throughout the body instead of defined endocrine glands
[29][74].
Figure 34. Mechanisms of hormonal actions.
Table 13. Classification of hormones.
3. Examples of Repositioned Hormones for Bone and Periodontal Tissue Engineering
As previously stated, GFs-based therapies are costly and may cause side effects and immunological reactions in certain individuals. To counteract these disadvantages, various hormones have been designed and tested as viable replacements to growth factors. Hormones are inexpensive to produce, can be readily designed and manufactured, and have little immunogenicity due to their flexibility
[30][75].
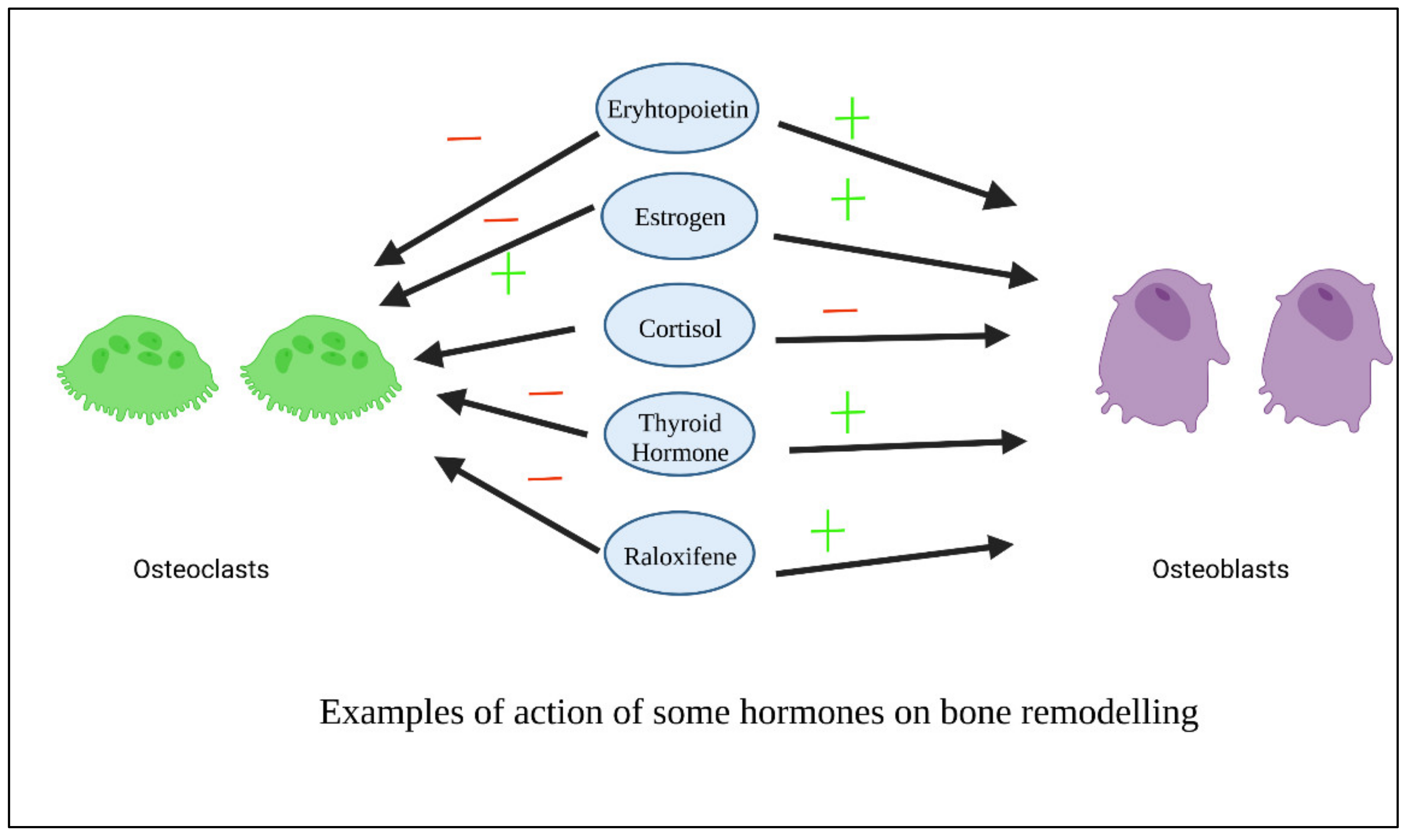
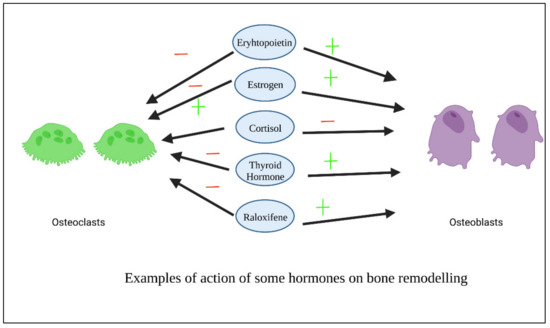
Figure 45 shows examples of the action of some hormones on osteoblasts and osteoclasts.
Figure 45. Examples of action of some hormones on osteoblasts.
3.1. Thyroxin
Thyroxin is an essential hormone that performs a range of physiological tasks in the human body. One of them is its capacity to stimulate angiogenesis through a variety of methods
[31][88]. By stimulating integrin v3, thyroxin promotes the production of mediators of angiogenesis
[32][89]. Thyroid hormones also influenced cellular metabolic reactions and cell growth
[33][90]. Chitosan/collagen-based thyroxin-loaded hydrogels have a neovascularization capability, which suggests that they might be useful materials for prospective tissue engineering applications
[31][88]. Chitosan composite enclosed with varying doses of thyroxin were demonstrated to be biocompatible, and these hydrogels with pro-angiogenic activities have a high promising applications in periodontal regeneration
[34][76]. In comparison to chitosan, thyroxin-containing membranes demonstrated significant revascularization and rapid wound healing in rats
[35][91].
3.2. Oxytocin
Oxytocin (OT) is a fundamental anabolic hormone found in animals during breastfeeding that also has local impacts on bone turnover in addition to the systemic endocrine route
[36][92]. This hormone improves bone production by favorable control of osteoblast development, osteoclast activities, and overexpression of bone morphogenic protein 2 (BMP2)
[37][38][93,94]. Despite oxytocin being researched in a variety of medicinal applications, its influence on in situ osteogenesis has not been explored, most likely because of its limited half-life and instability versus hydrolysis
[39][95]. The impact of this hormone is only temporary in the absence of an adequate carrier and encapsulation technique, and the physicochemical stabilization cannot be preserved over the bone healing period. Thanks to their unique features, poly (D, Llactide coglycolide) PLGA copolymers have been used as local drug carrier for different types of biomolecules
[40][96]. Sustained release micro spherical oxytocin hormone in a polymeric hydrogel scaffold mixed with biphasic calcium phosphates combination promotes bone repair in the rat calvarias
[41][97]. Furthermore, OT-loaded b-TCP increases osteogenesis in rats with calvarias bone defects via an osteoinductive mechanism of action
[42][77]. In vitro, OT increased PDLSC proliferation, aggregation, and osteogenic differentiation. Additionally, OT’s influence on osteogenic development was driven by the ERK and AKT pathways. As a result, OT has the potential to be used in periodontal regeneration
[43][98].
3.3. Dexamethasone
Dexamethasone (DEX) has been demonstrated to enhance osteoblast development in vitro and bone tissue creation in vivo by enhancing osteoblast-related gene transcription
[44][45][99,100]. DEX has long been employed as an osteoinductive factor due to its excellent integrity as well as osteogenesis
[46][47][101,102]. High DEX concentrations, on the other hand, would inhibit osteoblast growth and create hazardous adverse effects
[46][48][101,103], As a result, its additional functional applicability in bone tissue engineering is limited. Thus, prolonged release of DEX is essential to maximize effectiveness while minimizing negative effects on bone regeneration. Porous bio composite matrices comprise the chitosan-alginate-gelatin scaffold in addition to the accumulation of calcium phosphate and DEX-loaded nano silica. Doping was manufactured and demonstrated increased growth and osteogenesis in rats, suggesting that they might be extremely good as potential local insertable frameworks for possible uses in bone tissue engineering
[49][78]. Dexamethasone (DEX) has been demonstrated to initiate bone marrow differentiation as well as guide cells toward maturation
[50][51][104,105]. Injectable hydrogels loaded with dexamethasone have a promising potential as an injectable drug-depot for bone repair therapy in cases of chronic inflammation
[52][106].
3.4. Androgens
In males, testosterone is the major sexual hormone and anabolic factor. In humans, testosterone is crucial in the male sexual organs, for example the testes, as well as in the promotion of secondary sexual traits such as increased muscular and bone mass
[53][107]. PLGA-coated pericardial inserts or membranes combining topical gradual administration of supplementary quantities of testosterone and alendronate may be a viable approach for stimulating in situ osteogenesis, leading to enhanced implant osseo-integration and repair of bone defects and fractures
[54][79]. In mice, testosterone delivered with a scaffold has similar effects to the Bone Morphologic Protein-2 in enhancing bone regeneration
[55][108].
3.5. Parathyroid Hormone (PTH)
The endogenous parathyroid hormone is a critical mediator of bone remodeling as well as a crucial regulator of calcium-phosphate equilibrium. This hormone promotes bone formation by activating numerous mechanisms involved in stem/preosteoblast cell osteo differentiation. Inhibiting osteoblast apoptosis can also increase the quantity of osteoblasts. PTH causes osteoblasts to release a number of growth factors, and it causes osteocytes to produce less sclerostin and DKK, two anti-osteoclastic and Wnt signaling inhibitors. Furthermore, PTH may indirectly trigger osteoclasts to accomplish bone resorption. PTH stimulates osteoblast RANKL synthesis and increases RANKL binding to osteoclast surface receptors, resulting in osteoclast activation
[56][109]. The amount and duration of PTH exposure influence bone production (anabolism) and bone resorption (catabolism). Constant and high hormone dosages promote bone breakdown, whereas minimal and inconsistent levels promote osteogenesis and increased mineral density
[57][110].
3.6. Insulin
Insulin is a hormone which affects energy production and balance, as well as being an important part in bone formation metabolism. Skeletal anomalies linked to Diabetes type I can be cured with insulin treatment
[58][59][116,117]. Clinically, it is frequently noted that insulin shortage increases the possibility of fracture. The use of insulin therapy dramatically boosted bone formation in patients with type 2 diabetes, which can minimize the risk of fracture
[60][61][118,119]. Insulin/IGF-1 has been proven in vivo to induce angiogenesis and give nourishment for bone growth.
[62][63][64][120,121,122]. Insulin can successfully enhance local skull bone growth in the mouse skull by raising the quantity of bone forming cells and the surface area of the osteoid
[65][123], and has the ability to control osteoclastic activity
[66][124]. In recent years, research has discovered that IGF-1 can also influence the formation and maturation of osteoblasts, hence increasing bone repair
[67][125]. Given the success of nanoparticles in drug loading, a variety of insulin carriers have been innovated, which could be breakthroughs in bioengineering technology
[68][126]. In another study, insulin-loaded poly lactic-co-glycolic-acid (PLGA) Nano spheres were incorporated into nano hydroxyapatite/collagen (nHAC) scaffolds, where insulin was successfully distributed from the nano spheres and aided bone regeneration in significant size impairments in the rabbit mandible
[69][81]. Furthermore, insulin-encapsulated PLGA microspheres greatly enhanced the insert’s stability in rabbits at Week 4, indicating that it is possible to lower the implant’s early failure rate without affecting serum biochemical markers
[70][127]. New bioactive injectable composites loaded with insulin have been developed and might be used to treat bone defects, notably as an economic promotion/substitute to BMP-2 approaches
[71][128]. Local insulin infiltration at the implant–bone contact has the potential to have significant therapeutic ramifications by spontaneously increasing the effectiveness of oral implantation in diabetic rats
[72][129].
3.7. Estrogen
Estrogen is a natural steroidal hormone that regulates bone mass and maintains bone tissue balance. The estrogen’s activity is directly connected to the regulation of osteoblast proliferation and differentiation. In addition, estrogen reduces apoptosis in osteocytes and osteoblasts while inducing apoptosis in osteoclasts. By decreasing the synthesis osteoclastic mediators, estrogen reduces the creation of active osteoclasts. Moreover, it increases the creation of osteoprotegerin by osteoblasts and osteocytes (OPG)
[73][74][130,131]. 17-estradiol (E2) is the most powerful hormone in the body system, and it adheres to estrogen receptors (ERs) in both bone cells and MSCs. Estradiol can encourage MSCs to differentiate into osteoblasts and improve osteogenesis by boosting the expression of BMP-2, TGF-1, and IGF-1
[75][132]. Estrogen activity causes bone remodeling to be balanced and bone metabolism to be modulated. As a result, estrogen deprivation reduces osseous density, raises the possibility of osteoporotic fractures, and causes bone loss
[76][133]. Systemic estrogen treatment can help reduce osteoporotic fractures in postmenopausal women. Accumulation in organs, on the other hand, generates negative consequences, for example, cardiovascular disease and breast cancer
[77][134]. A controlled release to administer the lowest therapeutic dosage while avoiding systemic adverse effects may be a desired method for extending estrogen clinical uses. Various tissue-engineering technologies have been investigated in order to create local delivery for an osteoporotic bone fracture. Nano materials have recently been identified as an excellent choice for the transport of biomolecules. 17-estradiol (E2) was put into a nano fibrous matrix, which demonstrated improved cell growth and osteoblast development mediators
[78][84].
3.8. Selective Estrogen Receptor Modulators (SERMs)
Selective estrogen receptor modulators (SERMs) are non-steroidal compounds that have estrogenic actions on the bone, vascular system, and lipid profile, while also having anti-estrogenic effects on the breast and uterine
[79][80][137,138]. Through an estrogenic action on the skeletal structure, they promote endochondral ossification, bone production, and callus remodeling
[81][139]. By reducing osteoblast and osteoclast bone turnover, selective estrogen receptor modulators decrease bone degradation and lessen the fracture probability
[82][83][140,141]. Several SERMs are now being used in clinical settings, including Raloxifene, Tamoxifene, bazedoxifene, Lasofoxifene, Ospemifene, Arzoxifene, Droloxifene, Idoxifene, and Fulvestrant
[84][85][142,143]. Tamoxifen is a therapy for breast cancer that reduces osteoclast-mediated bone resorption
[86][87][144,145]. Both raloxifene and bazedoxifene are SERMs that have been demonstrated to reduce bone resorption activity in postmenopausal osteoporosis patients
[83][85][88][89][141,143,146,147] and have been utilized to keep bone fragility fractures at bay. SERM binding to estrogen receptors (ERs) modifies the receptor’s structure or capacity to form a combination with co-regulators, altering their expression levels
[90][91][92][93][94][148,149,150,151,152].
3.9. 1, 25(OH) 2 Vitamin D3
Vitamin D is a fat-soluble hormone that governs bone development and strength and helps to maintain calcium-phosphorus proportions. Scientific proof suggests that vitamin D plays an autocrine function in bone production, mineralization, and degeneration. 1, 25(OH) 2 D3 influences osteoblastic protein production via the (MAPK) ERK1/2 system
[95][96][158,159]. Many studies have demonstrated that vitamin D has a high capability in both osteoinduction and odontiinduction. At modest doses of this chemical, the expression of OCN, OPN, DSPP, DMP-1, and bone mineralization has enhanced
[97][160]. Bordini et al. created a scaffold loaded with 1 nM 1, 25-dihydroxy vitamin D3. They discovered that vitamin D3 can boost odontoblastic marker expression
[98][161].
A cellulose/hydroxyapatite/mesoporous silica scaffold was created and supplemented with vitamin D3 in a similar work. In vitro research revealed that vitamin D3 might improve cell adhesion and proliferation (MG63). Furthermore, the ALP activity and calcium accumulation assays validated the synergistic effects of hydroxyapatite and vitamin D
[99][162].
3.10. Melatonin
Melatonin’s (ML) involvement in hard tissues has gotten a lot of attention
[100][101][163,164]. The indoleamine ML (N-acetyl-5-metoxy-tryptamine) is produced and released by the pineal gland in a circadian rhythm
[102][165]. Melatonin is also produced in possibly all organs in numbers of orders of magnitude greater than in the pineal gland and bloodstream
[103][166]. ML may be implicated in the formation of hard tissues such as bone and teeth
[104][167]. ML stimulates alkaline phosphatase activity and tissue mineralization
[105][168]. As previously indicated, ML has been employed for its anti-inflammatory, antioxidant, and free-radical-scavenging qualities
[106][107][169,170] and cytoprotective properties
[108][109][171,172]. When there is a large quantity of ML, the generation of inflammatory mediators decreases via modulating the NFkB activity, which contributes to the signaling route.
While the favorable benefits of ML on periodontal regeneration have been proven in gingival fibroblasts as well as in experimental animals, more research is needed.
[108][171]. ML has a circulation half-life of around 23 min
[110][173]. As a result, a few writers have advocated for the use of vehicles in ML to slowly release it and enhance the duration of action in tissues. Steady ML release using poly-lactic-co-glycolic acid micro particles has been demonstrated to convert human mesenchymal stem cells into osteoblasts. Melatonin-loaded chitosan (ML-CS) micro particles (MPs) can modulate Mel release over time, accelerating osteogenic differentiation of preosteoblast cells in vitro
[111][86]. Local administration of 2 mg melatonin gel is a viable treatment method for effective bone and PDL regeneration in diabetic rats
[112][174]. Melatonin has the potential to be a promising implant coating. When powdered melatonin was applied to implant sites, it caused considerably increased bone growth and bone mineralization in canines in comparison with control groups
[113][175]. Melatonin improves the osteogenic properties of bone grafts around dental implants in canines
[86][144]. The findings of a 3-month clinical investigation demonstrate that melatonin may be therapeutically useful in improving the Osseo integration of dental implants
[114][176] Novel ML delivery methods, such as ML microspheres, have demonstrated tremendous potential for application in regenerative medicine and dentistry, particularly in bone-grafting techniques, to stimulate new bone growth
[115][177].
3.11. Erythropoietin
Erythropoietin (EPO), a glycoprotein that is generally known as an important stimulant of erythropoiesis, is released by kidneys in adult animals and in the liver during intrauterine life
[116][178]. Erythropoietin (EPO) is a glycoprotein hormone with a low molecular weight (30–36 kDa) that stimulates erythropoiesis. RhEPO received FDA approval in 1989, and it is now used to treat anemia caused by renal insufficiency, chemotherapy, bone marrow transplant, and AIDS
[117][118][119][179,180,181]. EPO has non hematopoietic cellular receptors in skin, and the presence of EPO receptors on endothelial cells
[92][93][150,151] and macrophages has been documented
[120][121][182,183] in macrophages
[122][184], fibroblasts, and mast cells
[123][124][185,186]. Erythropoietin and its ligands are found in both the central and peripheral nervous systems
[125][126][187,188]. Erythropoietin boosts anti oxidative enzyme synthesis, antagonizes glutamate cytotoxicity, influences neurotransmitter release, and induces neo angiogenesis
[127][189]. Unlike previously held beliefs that EPO was exclusively beneficial in the formation of erythropoiesis, Epo has been shown to have multiple effects, such as tissue modulation in a variety of cell types
[128][129][130][131][190,191,192,193]. There is growing evidence that EPO plays biological roles in tissues outside than the hematopoietic system, which has sparked major experimental interest. EPO is a tissue-protective hormone that promotes wound healing in a variety of damage scenarios such as tissue/organ inflammation
[132][194]. The healing of skin lesions in rats with intentionally induced diabetes is expedited by the local administration of recombinant human EPO to the wounds, which stimulates angiogenesis, reepithelialization, and collagen deposition, while inhibiting inflammatory process and apoptosis
[133][195]. Fibronectin supplements EPO’s positive effects on wound healing in diabetics (FN). FN promotes the establishment of the preliminary wound matrix and keeps it from dissolving
[134][196].
3.12. Calcitonin (CTN)
Calcitonin (CTN), a hormone secreted by par follicular cells (C cells) in the thyroid gland, is crucial in bone maintenance and calcium metabolic control
[135][205]. CTN binds to osteoclasts only in bone tissues, demonstrating the greatest expression of calcitonin receptor (CTR), and triggers osteoclast activity to cease
[135][136][205,206]. CTN, according to Granholm et al., suppresses osteoclast development in mouse hematopoietic cells through modulating RANK signaling
[137][207]. CTN has also been used to treat hypercalcemia from cancer and postmenopausal osteoporosis
[138][208]. In rats with periodontitis, local injection of CTN reduced alveolar bone resorption through controlling osteoclast activation
[139][87].