Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Sirius Huang and Version 3 by Raúl Poutou-Piñales.
Antibiotics are the therapeutic option for countless infections treatment; unfortunately, they are the second most common group of drugs in wastewaters worldwide due to failures in industrial waste treatments and their irrational use in humans and animals. Several techniques have been assayed for the degradation and mineralisation of antibiotics to reduce the environmental impact; strategies focused on physical, chemical, biological, and combined process design for degradation.
- antibiotics
- laccases
- wastewaters
- antimicrobial resistance
- treatment
1. Overviews of Degradation of Antibiotics
Antibiotics are ionisable and can be in the environment as neutral or charged (negative or positive) species. These species have different chemical properties, hence different sorption and degradation mechanisms in soil [144][1]. Several biotic and abiotic factors influence the degradation rate of these compounds, which is why each can have a different half-life in soil, ranging from <1 to 3466 days [145,146][2][3]. Enzymatic hydrolysis is one of the most frequent abiotic degradation pathways. The β-lactams are more susceptible to this degradation than macrolides or sulphonamides, while quinolones and tetracyclines are susceptible to photodegradation. These differences are inevitably dependent on the chemical structure of each class of antibiotics [145][2].
Several techniques have been assayed for the degradation and mineralisation of antibiotics to reduce the environmental impact [147][4]; strategies focused on physical, chemical, biological, and combined process design for degradation [147,148,149,150,151,152,153][4][5][6][7][8][9][10]. Physical and chemical methods include sedimentation, filtration, and oxidation using chemical compounds, UV or Vis light, ultrasound, and ions. Biological ones include the use of microorganisms and or their enzymes [148,149,150][5][6][7].
Unfortunately, many conventional wastewater treatment plants worldwide are not appropriate for removing highly polar micropollutants, such as antibiotics [148][5]. These plants usually use methods, such as filtration to remove larger solids, whereby wastewaters pass through a granular media, such as sand, charcoal, diatomaceous earth, or activated carbon; subsequently, removing the smaller solids by mechanisms, such as particle retention, electrostatic attraction, or adsorption. These wastes are not degraded and concentrate in a solid phase, generating a new waste. Sometimes, these sludges with antibiotic concentrations ranging from ng kg−1 to 100 mg kg−1 are used as fertilisers, indicating that the problem has not been solved but shifts the contaminants to other environments [146][3]. Chemical-assisted sedimentation allows the removal of suspended solids, for which chemical compounds, such as lime (CaO), potassium aluminium sulphate (KAl(SO4)2 12H2O), or iron salts (FeSO4) facilitate the precipitation of solids and their subsequent removal [148][5]. In addition, conventional wastewaters treatment plants use aerobic or anaerobic processes to remove organic matter [147,149][4][6].
Advanced Oxidation Processes (AOP) have been proposed for industrial wastewaters treatments, such as photocatalysis with TiO2 using heterogeneous semiconductors, UV photolysis, Photo-Fenton, or electro-Fenton.
Another study used the ferrous ion (Fe(II)-activated decomposition of persulphate (S2O8−2), for remediation of groundwater containing antibiotics. Hydroxyl (HO−) and sulphate (SO4−) radicals were the main reactive species, and therefore, responsible for the degradation of both Ciprofloxacin (CIP, 95.6%) and Sulfamethoxazole (SMX, 35%) [154][11].
Chlorinated compounds are frequently used in drinking water treatment plants as disinfectants or oxidising agents. However, the reaction of chlorine with aromatic rings, neutral amines, and double bonds generates halogenated compounds, which may be carcinogenic [147,149][4][6].
1.1. Biotic Degradation of Antibiotics
Several authors have studied the biodegradation of antibiotics, Jiang et al. (2010) evaluated the degradation of cephalosporins in the surface water and sediments of Lake Xuanwu, China, showing that abiotic hydrolysis (for Cephradine RAD, Cefuroxime CXM, and Cefepime FEP) and direct photolysis (for Ceftriaxone CRO) were the main mechanisms of cephalosporin removal in the surface water of the lake; while aerobic and anaerobic biodegradation removed cephalosporins in the sediment [155][12].
The degradation of antibiotics in soil depends on physicochemical characteristics [145,146][2][3]. Antibiotics accumulate in the soil’s upper layers, which act as a sink; these compounds are washed away by surface water and then leach into groundwater. The transport of antibiotics depends on the chemical properties of each antibiotic and the soil; soil organic carbon content, clay content, texture, pH, and ionic strength can alter the degree of sorption [144,156][1][13]. The sorption of antibiotics in the soil can modify their mobility, ecotoxicity, and bioavailability for microbial degradation; with low sorption, there is an increased risk of leaching and increased risk of contamination to groundwater [144,157][1][14].
Different classes of antibiotics, such as tetracyclines, sulphonamides, and macrolides, have been quantified in soils, sludge and manure. In sludge, for example, concentrations of Ciprofloxacin (CIP), Norfloxacin (NX) and Tylosin (TLS) have been reported with an average value above 1000 μg kg−1. Other antibiotics, such as Tetracycline (TC) and Oxytetracycline (OXT) appeared in sludge with values above 10 μg kg−1 dry matter. Additionally, antibiotics in manure have been identified, with concentrations between 100 and 1000 μg kg−1 dry matter. The manure or sludge application as fertiliser can facilitate the spread of resistant bacteria in the soil, particularly human pathogenic bacteria. However, their microbial degradation has been also reported [146,156,157,158,159,160,161,162][3][13][14][15][16][17][18][19].
Lin and Gan (2011) studied arid soils concerning the capacity of sorption and persistence of Sulfamethoxazole (SMX) and Trimethoprim (SXT). Sulfamethoxazole (SMX) sorption was minimal sorbed concentration (Cs = 0.973 µg kg−1), indicating a high risk of leaching, while Trimethoprim (SXT) showed moderate to high sorption (Kd = 7.4242 L kg−1). The degradation of antibiotics depended on microbial activity, oxygen in the soil, soil type, and antibiotic characteristics [157][14]. Pan and Chu (2016) obtained similar results; antibiotics belonging to the sulphonamides presented low sorption measured as the adsorption or soil distribution coefficient (Kd = 1.365 L kg−1), implying a higher risk to groundwater. In contrast, Tetracycline (TE) obtained the highest sorption (Kd = 1093 L kg−1) value, which would mean a lower risk of groundwater contamination with leachates. Furthermore, the antibiotics evaluated were susceptible to microbial degradation under aerobic and anaerobic conditions. They also showed that, at high antibiotic concentrations, degradation processes are slowest, and their persistence in the soil is prolonged [144][1].
Microorganisms, such as Stenotrophomonas maltophilia, Sphingobacterium sp., Klebsiella sp., Bacillus sp., Shewanella sp., and Trichosporon mycotoxinivorans participate in microbial degradation of tetracyclines. Several proposed degradation routes are in Stenotrophomonas maltophilia; reactions, such as denitromethylation, decarbonylation and deamination have been identified. Likewise, in the degradation route by Klebsiella sp., oxidation, hydrolysis ring-opening, decarbonylation, deamination and demethylation reactions are included. Whereas the degradation pathway by the fungus Trichosporon mycotoxinivorans includes epimerization, dehydration, and proton-transfer pathway reactions [158][15].
However, Alexy et al. (2004) evaluated the degradation of antibiotics using the closed bottle technique, which contained a mineral medium, inoculum, the antibiotic, and sodium acetate. Under these conditions, neither Amoxicillin (AMC), Benzylpenicillin (PG), Ceftriaxone (CRO), Cefuroxime (CXM), Chlortetracycline (CTC), Clindamycin (CM), Erythromycin (E), Gentamicin sulphate (GM), Imipenem (IPM), Ofloxacin (OFL) or Sulfamethoxazole (SMX), were biodegraded less than 60% in 28 days. Authors also claim that their removal from the environment by other abiotic mechanisms, such as temperature (thermal treatment) and light (photo treatment) is possible [162][19].
On the other hand, in a composting process of sludge or manure, antibiotic removal can range from 17 to 100% [146,163][3][20]. Composting is one of the techniques used for antibiotic and resistance gene removal in sludge or manure. The persistence and mobility of antibiotics in compost depend on their chemical properties, concentration, and physicochemical conditions, including pH, temperature, total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), and metal content. However, the removal efficiency of residual antibiotics, and antibiotic resistance genes, is still very low [163][20].
1.2. In Situ Chemical Oxidation (ISCO) of Antibiotics
In situ chemical oxidation is a removal strategy for different compounds, based on the addition of oxidising chemical compounds into the soil and groundwater to remove organic pollutants. Several authors have studied the degradation of antibiotics based on this strategy: thermo-activated persulfate oxidation [164][21], peroxymonosulfate oxidation [165][22], ozone oxidation [166][23], or ozone-based advanced oxidation process [167][24]. Wu et al. (2020) used a peroxymonosulfate activation support (amorphous CoSx cages advanced oxidation catalysts) to detect the degradation of Tetracycline (TE, 90%) and Ciprofloxacin (CIP, 90%). They also identified the intermediates and possible degradation routes after peroxymonosulphate activation, in which reactive species (SO4, O2, and OH) formed, and oxidised Tetracycline (TE) in reactions, such as deamidation, hydroxylation and the ring-opening reaction. They suggested that some intermediates (SO4·−/O2·−/·OH) could be mineralised (H2O and CO2) [168][25].
Guo et al. (2013) used Co3O4-catalyzed peroxymonosulfate oxidation based on sulphate radicals for Amoxicillin (AMC) degradation. This process is based on the generation of sulphate radicals through Co3O4-mediated peroxymonosulphate (PMS) activation. The authors observed a decrease in Chemical Oxygen Demand (COD) of 91.01% [169][26].
1.3. Photocatalytic Advanced Oxidation Processes (AOP) for Heterogeneous Degradation of Antibiotics
AOP are advanced oxidative processes based on the generation of highly reactive hydroxyl (OH*) radicals. These radicals come from oxidising agents, such as hydrogen peroxide (H2O2) or ozone (O3), usually using catalysts, such as titanium dioxide (TiO2) and UV light, which is known as heterogeneous catalysis [170][27]. Several authors reported AOP removal rates (>90%) for penicillins, sulphonamides, phenicols, β-lactams, tetracyclines, and fluoroquinolones [171,172,173,174,175][28][29][30][31][32]. However, the toxicity may not be totally reduced, and some intermediates generated could be more toxic than the original compounds [147,176][4][33]. On the other hand, AOPs combined with biological treatments (gamma irradiation, ionising irradiation integrated with activated sludge, a biological treatment combined with flocculation and ultrafiltration) have shown high removal yields (close to 100%) of toxicants and recalcitrants [174,177][31][34].
1.3.1. Photodegradation of Antibiotics Using TiO2 Based Heterogeneous Semiconductors
Photocatalysis using semiconductors is an indirect photocatalytic AOP that uses TiO2 electrodes for organic and inorganic compound oxidation–reduction reactions. TiO2 is non-toxic and has a high catalytic activity; however, its catalytic activity is attenuated by solar irradiation; therefore, other irradiation techniques, such as ultraviolet light (UV) have been applied [170,178][27][35].
Some authors have reported antibiotic removal rates higher than 80%, under certain specific conditions (radiation intensity, composition, concentration of the hybrid material, the matrix used, pH and temperature), in the degradation of Ciprofloxacin (CIP, 88%), Gatifloxacin (GAT, 96%), Levofloxacin (LVX, 96%), Tetracycline (TE, 83%), Penicillin (P, 90%), Sulphonamide (SUL, 97%), Sulfamethoxazole (SMX, 80% mineralisation), Ampicillin (AM, 98%) and Erythromycin (E, 90%) [170][27]. Others have evaluated the degradation of penicillins (Amoxicillin AMC, Ampicillin AM, and Cloxacillin CLO) by photocatalysis with TiO2 and H2O2, finding 100% degradation [179][36].
Degradation of Amoxicillin (AMC), Ampicillin (AM), and Cloxacillin (CLO) using ZnO photocatalysis reported rates above 80% [180][37]. Zheng et al. (2018) utilised a graphitised mesoporous carbon (GMC)-TiO2 nanocomposite, adsorbing the antibiotics efficiently. Subsequently, the photocatalysts and reactive species (hydroxyl radical) were contacted for antibiotics degradation; 15 mg L−1 of Ciprofloxacin (CIP) was fully mineralised (CO2 and H2O) in 1.5 h [181][38].
Abellán et al. (2009) by using several concentrations of TiO2, assayed Sulfamethoxazole (SMX) and Trimethoprim (TMP) removal; degradation >90% was observed for both compounds; optimal concentrations were 1.0 and 0.5 g TiO2 L−1, respectively. They also observed that SMX reduced its aromatic content, while TMP increased its aromatic content during the first three hours due to the formation of dimers; finally, the aromatic content decreased [182][39].
1.3.2. Photodegradation of Antibiotics Using Non-TiO2 Based Semiconducting Catalysts
Other catalysts than TiO2 have been used for antibiotics removal; for example, the hybrid material composed of co-modified NiS and MoS2 nanoflakes (g-C3N4-NiS-MoS2), synthesised by the direct mixing of graphitic carbon nitride with nickel salts and thiourea, has also used. Tetracyclines have been the most studied compounds using this catalyst, obtaining degradation rates >90% [170][27]. Several authors have evaluated different hybrid materials as catalysts for antibiotic degradati4n and have shown them to be efficient systems; using the Ppy-BiOI catalyst, 54% and 61% degradation was obtained for Chlortetracycline (CTC) and Tetracycline (TE), respectively. The hybrid material CQDs-Bi2WO6 degraded 87% of Ciprofloxacin (CIP) in 120 min., [178,183,184,185,186,187,188,189][35][40][41][42][43][44][45][46].
1.3.3. Photo-Fenton or Electro-Fenton of Antibiotics Removal
Fenton is an advanced oxidation process in which chain reactions mediated by a non-toxic catalyst (Fe2+), an acidic medium (pH = 2.8–3.0), and an oxidant (H2O2) take place for the formation of hydroxyl radicals (OH). Several studies address the degradation of Tetracyclines (TE), Norfloxacin (NX), and Penicillin (P) using this method, showing degradation rates >80% [170,190][27][47]. Likewise, the large-scale practical application of the Fenton process has disadvantages due to the large amount of ferric sludge it generates. This solid waste sludge is considered a potential hazard due to the adsorbed organic residues of the treated wastewaters. Therefore, special treatment is required, and solid waste disposal must be at specific locations [158][15].
1.4. Degradation of Antibiotics in Water by Plasma Treatment
Another technique used for antibiotic removal is the dielectric barrier plasma discharge (DBD); this technique generates oxidising species: radicals (H, O, OH) and molecules, such as H2O2 and O3 for the removal of pollutants in the air and water [190][47]. Plasma treatment has a strong oxidising capacity, high efficiency, and experimental ease and does not require exogenous reagents. Magureanu et al. (2011) evaluated the degradation of Amoxicillin (AMC, 100%), Oxacillin (OX, 100%), and Ampicillin (AM, 100%). Amoxicillin (AMC) was degraded after 10 min of dielectric barrier discharge plasma, while the other two antibiotics degradation took around 20 to 30 min [191][48].
Kim et al. (2013) showed the degradation of Lincomycin (LIN), Ciprofloxacin (CIP), Enrofloxacin (ENR), Chlortetracycline (CTC), Oxytetracycline (OXT), Sulfathiazole (STZ), Sulfamethoxazole (SMX), and Trimethoprim (SXT). The results indicated that in degradation by dielectric barrier discharge plasma, the degradation rates were >90% but depending on the amount of energy supplied, and each antibiotic showed a different degradability [192][49].
Li et al. (2020) also assayed Tetracycline (TC), Sulfadiazine (SD), and ciprofloxacin (CIP) degradation by non-thermal discharge plasma. All antibiotics showed different degradation rates, and 20 min after plasma treatment at 19 Kv, found degradation efficiencies of 93.3, 81.2, and 58.5% for TC, SD, and CIP, respectively. The clearance efficiency and mineralisation yield of Tetracycline (TE) were relatively higher than the other antibiotics [190][47].
Sarangapani et al. (2019) evaluated the degradation of Ofloxacin (OFX) and Ciprofloxacin (CFX) by the atmospheric cold plasma method. Such plasmas generate the chemical reactions responsible for the formation of reactive species. The degradation was 92% and 89% for OFX and CFX, respectively, reducing the activity of both antibiotics [193][50].
1.5. Cathodic Degradation of Antibiotics
Cathodic degradation is an emerging technology that has potential in wastewaters treatment for nitro compounds, aromatics, azo dyes and halogenated compounds removal. This technique provides electrons for reductive degradation of pollutants, is environmentally friendly as no chemicals are needed, requires low voltage application and is low-cost [194][51]. Kong et al. (2015) assayed the degradation of Nitrofurazone (NFZ, 98.71%), Metronidazole (MNZ, 98.21%), Chloramphenicol (CHL, 99.86%) and Florfenicol (FLO, 99.72%) by using a dual-chamber electrochemical reactor. The different cathodic potentials applied generated a high efficiency in the degradation of the antibiotics. Finally, the authors conclude that electrochemical reduction is promising as a pretreatment or advanced treatment of wastewaters containing antibiotics [194][51].
1.6. Temperature Degradation of Antibiotics
Several authors have studied the effect of different temperatures on antibiotic degradation. Lin et al. (2017) evaluated the temperature effect on the degradation of antibiotics in manure (one of the main reservoirs of antibiotics and resistant bacteria); they incubated pig and chicken manure at 30, 40, 50 and 60 °C for five days, identifying the presence of different classes of sulphonamides. In pig manure, they found Sulfadiazine (SDZ), Sulfadimethoxine (SDM), Sulfamethoxine (SMZ) and Sulfamethamonomethoxine (SMM); in chicken manure, they found Sulfadiazine (SDZ), Sulfadimethoxine (SDM), Sulfamethoxine (SMZ) and Sulfaquinolaxine (SQ). The temperature effect varies depending on manure origin and antibiotic class; at 60 °C, the residual concentration of antibiotics in pig manure decreased up to 2 mg kg−1. In contrast, the sulphonamides in chicken manure were the lowest at 30 °C (0.5 mg Kg−1); however, they increased at 40 °C (7 mg Kg−1) and then decreased continuously as the temperature increased [195][52].
Loftin et al. (2008) tested the degradation at different temperatures (7, 22, and 35 °C), pH (2, 5, 7, 9, and 11), and ionic strength (0.0015, 0.050, and 0.084 mg L−1) of Chlortetracycline (CTC), Oxytetracycline (OXT), Tetracycline (TE), Lincomycin (LIN), Sulfachloropyridazine (SCP), Sulfadimethoxine (SDM), Sulphathiazole (STZ), Trimethoprim (SXT), and Tylosin (TYL), observing that temperature and pH affected the degradation rate of CTC (22 °C, pH 9 ± 0.2), OXT (22 °C, pH 7 ± 0.2), and TE (35 °C, pH 9 ± 0.2), which increased with increasing temperature [150][7].
1.7. Sonocatalytic Degradation of Antibiotics
Sonocatalysis is a recently advanced oxidation process (AOPs) used for water treatment using ultrasound; this process induces acoustic cavitation “in situ” to remove contaminants in a liquid phase [196,197][53][54].
Cavitation is the formation, growth and implosive collapse of gas- or vapour-filled microbubbles (considered microreactors) resulting from the acoustic wave/rarefaction-induced compression in a liquid [197][54]. Due to the high temperatures (≥5000 K) and pressures (≥1000 atm) reached during the process, an oxidative environment is generated with highly reactive species (ROS), such as hydrogen free radicals H*, hydroxyl (*OH) and hydroperoxyl (HO2*) [196,197][53][54]; species capable of degrading recalcitrant contaminants, attacking organic molecules instantaneously [196][53].
De Bel et al. (2009) used sonolysis at 520 kHz and 92 WL−1 for the degradation of Ciprofloxacin (CIP), finding that the degradation rate is pH-dependent due to the degree of protonation and positive charges acquired by Ciprofloxacin (CIP) during treatment which favour ultrasonic degradation. In this study, the degradation rate increased, since the pseudo-first-order degradation constant increased almost four times when comparing the treatment at pH 3 (0.021 min−1), pH 7 (0.0058 min−1) and pH 10 (0.0069 min−1), obtaining a BOD/COD ratio of 0.06 to 0.60; 0.17 and 0.18, after 120 min of irradiation, respectively [198][55].
Sonochemical degradation of antibiotics has been promisingly successful due to TOC (Total Organic Carbon) reduction, COD (Chemical Oxygen Demand) removal and an increase in BOD5 (Biological Oxygen Demand) and BOD5/COD ratio. In this regard, Liu et al. (2021) provide an overview related to the removal efficiency (RE) of various antibiotics using different conditions, such as ultrasonic frequency (US, 20–600 kHz); electrical power input (PE, 60–860 W); sonication times (t, 20–300 min) by using removal processes, such as Sonication alone, Sonocatalysis, Sono/Fenton, Sono/PS, Sono/Photo, and Sonozonation. In general, summarized results by Liu et al. (2021) showed different removal efficiencies for antibiotics, such as Sulfamethazine (SM2), Oxacillin (OX), and Dicloxacillin (DCX), all of them with 100% RE; Sulfadiazine (SD, 90% RE); Cephalexin (CN), Ciprofloxacin (CIP), Penicillin G (PG), all of them with 52–66. 7% RE; Ofloxacin (OFL, 31% RE); Levofloxacin (LVX, <10% RE) [199][56].
However, the results reviewed by Liu et al. (2021) also showed that: (i) the antibiotic’s distance from cavitation bubbles is crucial, (ii) that removal efficiency (RE) is pH-dependent in some cases, (iii) that the hydrophobicity of the antibiotic is critical for some processes, (iv) that in some cases mineralization is not achieved, and (v) that molar volume is critical for reactions [199][56]; indicating that even some adjustments are necessary for the treatment of each molecule; however, the residuals to be treated are a mixture of these target molecules, among others.
In summary, the biotic, physical and chemical antibiotic degradation processes have obtained high elimination percentages (72.5–90%); however, only a few authors have reported the intermediates and possible degradation steps or sequence, because intermediates compounds generated vary according to the antibiotic degraded, the process (biological, chemical, physical, or combined) and the conditions used (pH, temperature or presence of chemicals) [200][57]. The identification of intermediate compounds resulting from antibiotic degradation, their toxicity, and residual antimicrobial activity is crucial to establishing the process efficiency; likewise, knowing the different degradation steps is the key to improving each treatment [201][58]. In this respect, Fan et al. (2019) proposed a degradation route of Cefazolin (CZ) from an advanced oxidation process with activated peroxymonosulfate (PMS); high molecular weight sulfoxide compounds were identified (Figure 1a) [202][59]. Some authors also have identified intermediates generated in the degradation of Cefazolin (CZ) by using oxidation reactions; Li et al. (2016) used permanganate (Mn (VII)) as an oxidant and identified five intermediates, of which one of these matches the compounds detected by Fan et al. (2019), (Figure 1b) [200][57]. Ghasemi et al. (2020) determined the intermediate compounds generated from the degradation of Cefazolin (CZ) from an electro Fenton (EF) process, identifying low molecular weight compounds, so the authors claim that their mineralisation using a biological treatment is possible (Figure 1c) [203][60].
On the other hand, the possible Cefazolin (CZ) degradation after photocatalysis processes have proposed; Gurkan et al. (2012) used UV light and TiO2, and the authors determined that the degradation of Cefazolin (CZ) occurs through intramolecular cleavage of lactam rings, thiadiazole, tetrazole, and subsequent reactions with -OH* radicals that transform the fragments into smaller species [204][61]. Figure 1d shows some of the intermediates generated. Chen et al. (2021) used a bismuth oxybromide (BiOBr) photocatalyst for the removal of Cefazolin (CZ) [205][62]; in this study, hydrolysis and oxidation reactions were identified (Figure 1e). Other techniques, such as sonocatalysis have also identified some intermediates (Figure 1f) [206][63].

Figure 1. Some intermediates generated and degradation steps of Cefazolin (CZ) from different techniques, m/z is the mass to charge ratio. (a) degradation of Cefazolin (CZ) in an oxidation process with activated peroxymonosulfate (PMS) [202][59]. (b) intermediates generated from an oxidation process with permanganate (Mn (VII)) [200][57]. (c) Intermediates generated from a Fenton process [203][60]. (d) Intermediates generated from photocatalysis with UV light and TiO2 [204][61]. (e) Intermediates generated from photocatalysis with bismuth oxybromide (BiOBr) [205][62]. (f) Intermediates generated from sonocatalysis [206][63].
Additionally, Levofloxacin degradation (LVX) has been extensively studied by different methods. Liu et al. (2021) and He et al. (2022) identified the intermediates generated from chemical oxidation using peroxymonosulfate; these authors, despite studying the degradation of the same molecule using the same technique, have found that the conditions surrounding the reactions crucially influence the generated intermediates to be different; however, both authors reported obtaining small molecules that can be mineralized [207,208][64][65]. Figure 2a shows some intermediates found [207,208][64][65]. Other studies have reported the degradation of Levofloxacin (LVX) by chemical oxidation using permanganate (Mn (VII)) (Figure 2b) [209,210][66][67]. Additionally, other authors evaluated the degradation of Levofloxacin (LVX) using Fenton and proposed the possible degradation steps (Figure 2c) [211,212,213,214][68][69][70][71].
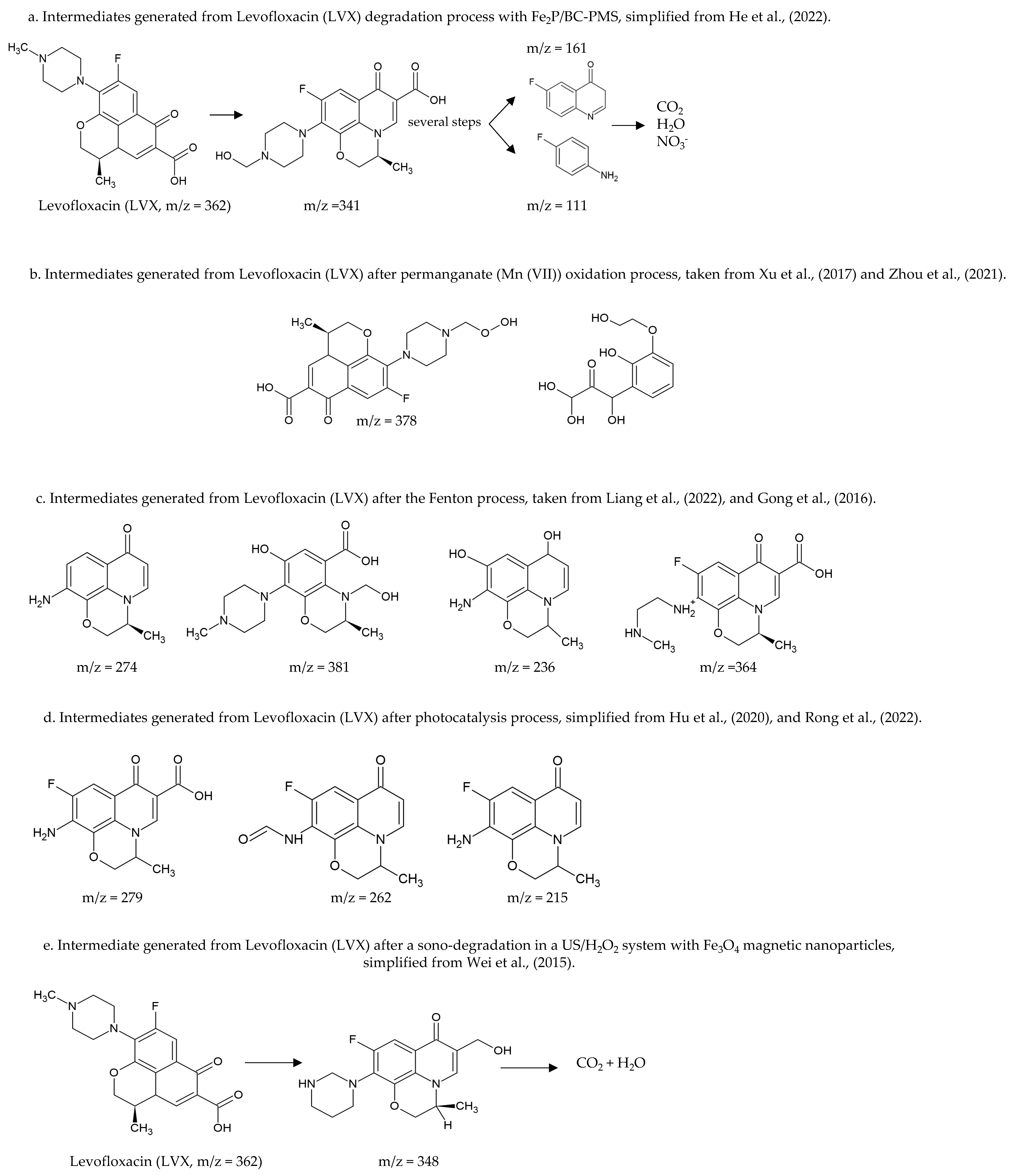
Figure 2. Some intermediates and degradation steps of Levofloxacin (LVX) by employing different techniques for degradation, m/z is the mass to charge ratio. (a) degradation pathway of Levofloxacin (LVX) in an oxidation process with peroxymonosulfate (PMS) [207,208][64][65]. (b) Intermediates generated from an oxidation process with permanganate (Mn (VII)) [209,210][66][67]. (c) Intermediates generated from a Fenton process [211,212,213,214][68][69][70][71]. (d) Intermediates generated from photocatalysis [202,215,216][59][72][73]. (e) Intermediates generated from sonocatalysis [217][74].
Moreover, other authors have evaluated the degradation of Levofloxacin (LVX) using photocatalysis under different conditions, generating different compounds (Figure 2d) [202,215,216][59][72][73]. Sonocatalysis also has been tested for the degradation of Levofloxacin (LVX); Wei et al. (2015) proposed the possible degradation steps to achieve the mineralization of the compound (Figure 2e) [217][74].
Table 1 summarises some biotic, chemical, physical and combined treatments for the degradation of various antibiotics.
Table 1. Types of treatments and techniques for antibiotics degradation, summary of degradation or removal percent.
| Types of Antibiotic Degradation | Techniques | Process or Materials | Antibiotics Reported | Degradation/Removal | References |
|---|---|---|---|---|---|
| Biotic | Hydrolysis | Lake sediment. | Cephradine (BAN), Cefuroxime (CXM), Ceftriaxone (CRO), and Cefepime (FEP). | Reporting removal of Ceftriaxone disodium (CRO) of 3% and Cefotiam dihydrochloride (CTM) of 7% in 28 d. | [155][12] |
| Microbial degradation | Bacterial suspensions of poultry manure and soil that produce humic acids. | Tetracycline (TE), Oxytetracycline (OXT), Chlortetracycline (CTC). | Removal between 88 and 75% in 15 min. | [218][75] | |
| Degradation attributed to a co-metabolic process | The strain was isolated from a reactor used for the treatment of aquaculture effluent. | Oxytetracycline (OXT), Ciprofloxacin (CIP). | Oxytetracycline (OXT) between 90.3 and 97.4 and Ciprofloxacin (CIP) were unable to degrade. | [159][16] | |
| Chemical | Heat activated of Persulfate | Aqueous solution at different pH. | Penicillin G (PG). | At pH 5 was removal 82.6 and at higher pH, the removal decreased. | [219][76] |
| Electrochemical oxidation | EC reactor with a built-in platinum counter electrode and Roxy potentiostat. | Ciprofloxacin (CIP), Norfloxacin (NX), Ofloxacin (OFL). | Reporting removal of Ciprofloxacin (CIP) of 90%, Norfloxacin (NX) of 62% and Ofloxacin 97.3%. | [220][77] | |
| Fenton process | The reaction was improving with H2O2, promoting catalytic production. | Thiazole sulphate, Tylosin (TLY), Ciprofloxacin (CIP), Amoxicillin (AMC) Cloxacillin (CLO), Tetracycline (TE). | Reporting removal between 77 and 97.1%. | [221][78] | |
| Physical | Adsorption | Biochar of waste sludge. | Tetracycline (TE), Sulfamethazine (SM2) | Weak adsorption using biochar could be overcome with the use of peroxymonosulfate. | [222][79] |
| ZSM-5 zeolite and zeolites nanocrystals. | Ciprofloxacin (CIP). | Removal between 54 and 90% according to material, time and antibiotic concentration. | [223][80] | ||
| Temperature | Increased temperature with incubation | Sulphonamide (SUL). | Minimum temperature of 60 ºC removing between 78.1 and 98.3 % on different sulphonamide antibiotics. | [195][52] | |
| Photodegradation | It was evaluated under a 125 W UV A-B-C (200–600 nm) irradiation. | Ciprofloxacin (CIP), Ofloxacin (OFL). | There is no high evidence of the degradation of antibiotics by exposure to UV light. | [224][81] | |
| Physico-chemical | Plasma treatment | Plasma reactor in coaxial configuration operated in pulsed mode. | Oxacillin (OX), Amoxicillin (AMC), Ampicillin (AM). | Oxacillin and Amoxicillin (AMC) had >90% conversion and Ampicillin (AM) was 29%. | [191][48] |
| Photocatalysis | Solarbox 1500 photoreactor produced by an air-cooled xenon lamp. | Ampicillin (AM), Enrofloxacin (ENR), Tylosin (TLY), Vancomycin (VA), Clindamycin (CM), Trimethoprim (SXT), Zetronidazole, Sulfadiazine (SD), Doxycycline (DO), Oxytetracycline (OXT). | The removal of antibiotics by photocatalytic oxidation was between 40 and 100% with the exception of Clindamycin (CM) which was not removed. | [225][82] | |
| Quartz reactor with a 20 W lamp and irradiation of 2300 μW/cm2. | Ciprofloxacin (CIP), Ofloxacin (OFL). | Removal of ~70%. | [226][83] | ||
| Advanced oxidation processes (AOP) | Oxidation with agents such as hydrogen peroxide, ozone, titanium dioxide and UV light using semiconducting materials, such as TiO2, SnO2, CeO2, and ZnO as catalysts. | Penicillins, Sulphonamides, Phenicols, β-lactams, Tetracyclines, Fluoroquinolones | Reporting removal with different efficiency ranges between 78 and 100%. | [227][84] |
2. Enzymatic Degradation of Antibiotics
For several years, the biodegradation of antibiotics by microorganisms, such as bacteria and fungi has been carried out. For example, aerobic heterotrophic bacteria, such as Actinobacteria or Proteobacteria can transform sulphonamides (to N4-acetylsulfamethoxazole) or even mineralise them (CO2 and H2O). However, in some antibiotics, only minor changes of the molecule occur without changing the activity, while for others, intermediaries are transformed back to the parent form; as occurs in the transformation of sulphonamides to N4-acetylsulfamethoxazole, which can be reversible [228][85]. In addition, the ability to degrade antibiotics is limited in some bacteria by environmental conditions [228][85].
Some techniques use enzymatic degradation under controlled conditions for wastewater treatment, such as the activated sludge bioreactor (ASB), in which pollutants are submitted to aeration to promote the growth of bacteria that degrade toxic organic substances [229][86]. The ASB is the most widely adopted system for biological wastewater treatment; however, this method is not for antibiotics removal; for which the concentration of antibiotics removed can vary between classes [230][87].
On the other hand, the use of membrane bioreactors (MBR) is one of the alternatives for the removal of antibiotics, such as Ciprofloxacin (CIP), Erythromycin (E), Tetracycline (TE), Ofloxacin (OFL) and Chlortetracycline (CTC) with removal rates higher than 90% [231][88]. In MBR, the mechanism for antibiotics removal is enzymatic biodegradation, which is a promising alternative to eliminating these contaminants due to its low energy requirements and high efficiency [232][89].
Oxidase enzymes have been the most studied enzymes in the degradation of antibiotics. Ligninolytic enzymes produced by fungi, bacteria, plants and algae, including laccases (Lac, E.C. 1.10.3.2), lignin peroxidase (LiP, E.C. 1.11.1.14), manganese peroxidase (MnP, E.C. 1.11.1.13), and versatile peroxidase (VP, E.C. 1.11.1.16), have been assayed for antibiotic removal, showing high degradation rates 72.5–90%) [4,233,234,235][90][91][92][93].
For example, Wen et al. (2019) evaluated the degradation of Oxytetracycline (OXT) by immobilising LiP enzymes from Phanerochaete chrysosporium on bentonite-derived mesoporous materials; the degradation was 95% [236][94]. Several authors have identified the intermediates generated in biotransformation, their toxicity and possible degradation routes. Tian et al. (2020) evaluated the degradation of Oxytetracycline (OXT) using laccase produced by the fungus Pycnoporus sp., in the presence of ABTS and Al3+, Cu2+, and Fe3+ ions. Authors showed that OXT was 100% degraded, and the antimicrobial activity was reduced after treatment. In this work, the degradation pathway includes the reactions of deamination, demethylation, and dehydration. Furthermore, seven intermediates in the degradation of OXT were identified [237][95].
Copete-Pertuz et al. (2018) evaluated the degradation of Oxacillin (OX), Cloxacillin (CLX) and Dicloxacillin (DCX) using the crude extract produced by the fungus Leptosphaerulina sp. The three antibiotics were 100% degraded, and laccase (Lac, E.C. 1.10.3.2) and versatile peroxidase (VP, E.C. 1.11.1.16) were the responsible enzymes for antibiotic transformation. The authors also demonstrated the lost antimicrobial activity of the three antibiotics and that intermediaries were shown to be non-toxic by the cytotoxicity assay [238][96].
Zhang et al. (2020) evaluated the degradation of antibiotics, such as Ampicillin (AM) and Tetracycline (TE) by immobilising the laccases produced by the bacterium Bacillus subtilis in a copper-trimic acid (Cu-BTC) framework. Without chemical mediators in the enzymatic treatment, a high degradation efficiency for both antibiotics (close to 100%) resulted. Eleven and fourteen intermediates, respectively, from Tetracycline (TE) and Ampicillin (AM) degradation, and the possible degradation routes, were identified. In addition, loss of antimicrobial activity and low ecotoxicity of the degradation products generated was demonstrated [67][97].
Sun et al. (2021) evaluated the degradation of tetracycline (TE) using the MnP enzyme. Eighty percent of Tetracycline (TE) was degraded within three hours, showing the antimicrobial activity of the transformation products from Tetracycline (TE) decreased over the reaction time. Seven compounds resulted from TE degradation and proposed a possible degradation route (demethylation, dimethylamino oxidation, decarbonylation, hydroxylation, and oxidative dehydrogenation) Sun [239][98].
In contrast, other authors have demonstrated the formation of toxic products due to the presence of chemical mediators. The effect of a mediator on oxidation depends on the laccase type, the substrate, the radicals formed, the mediator recyclability, and the laccase stability [4][90].
Weng et al. (2012) reported the degradation of Sulphadimethoxin (SDM) and Sulphamonomethoxin (SMM) using violuric acid (VLA), syringaldehyde (SIR), and 4-hydroxy benzyl alcohol (HBA) as mediators of enzymatic activity. Degradation of antibiotics using laccase in the presence of the mediators VLA or HBA generated degradation products of lower toxicity, in contrast to the treatments where the mediators were ABTS or SIR, which showed high toxicity [240][99].
Becker et al. (2016) reported that the mediator ABTS increased the degradation efficiencies of Sulfamethoxazole (SMX), Ampicillin (AM), and Trimethoprim (SXT), but decreased the degradation efficiency of Tetracycline (TE) and Oxytetracycline (OXT), furthermore, laccase was not able to transform Chloramphenicol (C) in the presence or absence of ABTS. The laccase combined with ABTS treatment, induced non-specific toxicity in some bioassays, involving the generation of transformation products or toxic radicals [4][90].
In some studies of antibiotic biotransformation, the degradation pathways, the intermediates formed, and the microorganisms and enzymes involved at each stage are still unclear. There are antibiotics widely used in humans and animals; however, their enzymatic degradation has not been assayed [231][88].
Chemical techniques for the removal of contaminating compounds have shown high efficiency in the degradation of antibiotics; however, they have high costs. In most of them, pollutant mineralisation is flawed, while more toxic degradation intermediates remain accumulated. For this reason, several authors have proposed that combined degradation and biodegradation processes may be the best solution for wastewater treatment. The enzymatic treatment can be pre- or post-chemical-treatment in conventional wastewater treatment plants (WWTPs) to increase biodegradability or degrade recalcitrant compounds that were not completely removed or mineralised at the conventional ones [231][88]. A higher removal rate of about 20 to 50% of hybrid processes appears to be more effective for recalcitrant antibiotics removal [229][86].
2.1. Laccases
Laccases (Lac, E.C. 1.10.3.2) are enzymes belonging to the cupredoxin superfamily, specifically the multi-copper blue oxidase family [241][100]. They are ubiquitous, found in fungi, bacteria, plants, and insects, organisms in which they play different roles. In fungi, laccases allow the depolymerisation of complex compounds, such as lignin [242][101] morphogenesis, pigment production, and defence against stress [243][102]. Different genera-producing laccases have been studied, such as white-rot fungi [244][103]. They are the most efficient organisms in lignin degradation until their mineralisation to CO2 and water. Lignin is the second most abundant biopolymer after cellulose and is one of the most recalcitrant natural compounds [245][104]. Lignin degradation, like that of other xenobiotic compounds, occurs due to the production of low specificity extracellular ligninolytic oxidoreductase enzymes, classified as phenol oxidases and haem-peroxidases [246][105]. Phenol-oxidase enzymes use oxygen (O2) as the final electron acceptor, whereas haem-peroxidases use hydrogen peroxide (H2O2) as the final electron acceptor. Phenol-oxidase enzymes include laccases, while haem-peroxidases include enzymes, such as lignin peroxidase (LiP, E.C 1.11.1.14), manganese peroxidase (MnP, E.C 1.11.1.13), versatile peroxidase (VP, E.C 1.11.1.16) and dyP-type peroxidases (E.C. 1.11.1.19) [242,247,248][101][106][107]. Additionally, there are accessory enzymes involved in lignin degradation, which produce hydrogen peroxide (H2O2) required by peroxidases, such as aryl alcohol oxidase (E.C. 1.1.3.7), glyoxal oxidase (E.C. 1.2.3.5) and glucose 1-oxidase (E.C. 1.1.3.4) [246][105]. The activity and ability of these enzymes to degrade compounds vary according to species and catalytic properties [248][107]. Laccase enzymes are the main enzymes involved in lignin degradation, and the rate of lignin degradation is related to the production of laccases. Currently, laccases are of great industrial importance as they can catalyse the oxidation of a large number of compounds and are also of great importance in the treatment of pesticides, explosives, wastewaters and synthetic dyes [243][102], and wastes generated by different industries, mainly paper, petrochemical and textile industries [249][108]. These waste compounds harm the environment; for example, synthetic dyes have complex chemical structures that are difficult to degrade and harm the environment and health, as they are toxic, carcinogenic and highly recalcitrant [250,251,252][109][110][111].
2.1.1. Laccase and the Degradation of Antibiotics
Laboratory Studies
Tetracycline’s broad action spectrum and low-cost favours its wide use in humans and animals. These antibiotics are frequently in wastewaters, and their degradation contributes to a significant decrease in antibiotic contamination. Therefore, Tetracycline (TE) degradation by using laccases has previously been reported. Zhang et al. (2020) obtained a degradation percentage of tetracycline (1 mg mL−1) close to 100%, using immobilised laccases from Bacillus subtilis [67][97].
Yang et al. (2017) immobilised laccases from Cerrena unicolor to assay the degradation of six antibiotics, Oxytetracycline (OXT), Tetracycline (TE), Trimethoprim-Sulfamethoxazole (SMX), Ampicillin (AM), Erythromycin (E), and Chloramphenicol (CHL), with or without ABTS as a mediator of enzyme reaction. The highest degradation (80%) was found on Tetracycline (TE) (100 µg mL−1), using 40 U mL−1 of laccases, pH 6.0 ± 0.2, 25 °C in 12 h [253][112].
Becker et al. (2016) evaluated the degradation of 38 antibiotics of different classes in an enzymatic membrane bioreactor by immobilising laccases produced by Trametes versicolor. In the presence of the mediator syringaldehyde, the degradation of 32 antibiotics (10 µg L−1) was greater than 50% in 24 h [4][90]. Similarly, Navada et al. (2019) evaluated the degradation of Chloramphenicol (CHL) (10 mg mL−1), a recalcitrant and thermostable antibiotic, using laccases produced by Trametes hirsuta in the presence of the mediator ABTS (0.25 mM) and observed 82% degradation in 48 h [254][113].
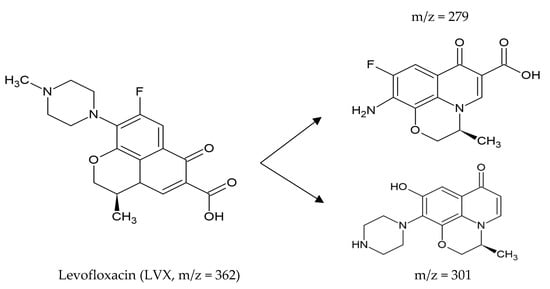
Najafabadipour et al. (2021) proposed degradation of Levofloxacin (LVX), generated from an enzymatic reaction with an osmotically stable laccase (E.C. 1.11.1.7) in a urea-containing solution, (Figure 3).
Moreover, some drugs are cytotoxic, mutagenic, teratogenic and carcinogenic, even at low concentrations; doxorubicin has been detected in hospital wastewaters, as it is excreted in urine from 3 to 10% and in faeces from 40 to 50% of the dose administered to the patient. Doxorubicin at concentrations of 0.05 μg L−1 causes DNA damage in Ceriodaphnia dubia. Kelbert et al. (2020) evaluated the degradation of doxorubicin using laccases from Trametes versicolor. The laccases degraded different concentrations (50, 250, and 500 μg L−1) of doxorubicin in 4 h; the assay conditions were 1800 UL−1 of laccases, pH 7.0 ± 0.2, and 30 °C [256][115].
Computational Studies for Antimicrobial Degradation Using Laccases
Bioinformatics tools facilitate the prediction of molecular mechanisms and the behaviour of enzymes against different ligands to obtain a prior estimation of their industrial potential use in bioremediation processes. Moreover, performing this computational simulation before experimental trials is economically convenient. The interaction enzyme-substrate can be studied through molecular docking [257,258,259,260,261,262,263][116][117][118][119][120][121][122] and molecular dynamics analyses [264][123]; for these studies, algorithms allow for calculating the electrostatic interactions of the amino acid residues in contact with the specific substrates (ligands) to be studied [265][124].
Computer tools allow the prediction of the properties of a molecule by homology modelling of its 3D structure. Structural knowledge of molecules is a prerequisite for molecular docking analyses, but this is the major limitation because, in most cases, the 3D structure of proteins is unknown, as they have not been purified and crystallised [257,259,260,261,262,263,264,266][116][118][119][120][121][122][123][125]; making it necessary to work with simulated models.
Experimental procedures for structure determination have certain disadvantages and limitations because, during the crystallisation of highly hydrated crystals, they can distort some regions as it is sometimes unclear whether an atom corresponds to the protein or the water molecule. Likewise, using techniques, such as Nuclear Magnetic Resonance (NMR) can determine the structure of proteins; however, these must be small proteins (less than 30 kDa), and difficult to crystallise [260][119]. Experimental structure determination is also expensive and time-consuming [267][126].
There are computational studies on interactions between laccases and different pollutants from the pharmaceutical industry. Singh et al. (2015) evaluated the interaction of laccases from Trametes versicolor with several pollutants from the pharmaceutical industry (Roxithromycin (RXT), Clarithromycin (CLR), Indomethacin (IMT), Bezafibrate (BZ), Metoprolol (MTL), Celiprolol (CPL), and Iopromide (IPD)), using ABTS as docking control molecule (−328.4 Kcal mol−1). Roxithromycin (RXT), Clarithromycin (CLR) and Iopromide (IPD) obtained lower energy values concerning the ABTS (−396.7, −380.5, −337. 7 Kcal mol−1, respectively), concluding that these contaminants are susceptible to degradation by laccases, contrary to the rest of contaminants that obtained higher energy values than ABTS, such as Indomethacin (IMT) (−294.0 Kcal mol−1), Bezafibrate (BZ) (−304.7 Kcal mol−1), Metoprolol (MTL) (−263.6 Kcal mol−1) and Celipropol (CPL) (−299.7 Kcal mol−1) [268][127].
Sakar et al. (2020) analysed the computational structure of laccases produced by Thermus thermophilus; the enzymes were characterised under physicochemical parameters, determining that they are mainly composed of negatively charged aliphatic amino acids, mostly valine and to a lesser extent cysteine. Additionally, due to the high value of the aliphatic index (41.29) and the presence of salt bridges in their structure, they are thermostable enzymes. In addition, identified random folding regions played a role in the flexibility and conformational changes of the protein. On the other hand, the Ramachandran analysis shows the statistical distribution of the torsion angles of the amino acid residues of a protein in the sterically favoured, allowed and not allowed regions; in this case, more than 90% of the amino acids were located in protein regions that were energetically favoured; demonstrating a high quality of the model. In addition, according to the negative value of hydropathy, they confirmed the hydrophilic nature of laccases and identified the binding sites and functional motifs [267][126].
Cárdenas-Moreno et al. (2019) computationally evaluated the interactions of Ganoderma weberianum laccases with different pharmaceutical compounds, including Tetracycline (TE) and Sulfixazole (SXZ). The authors modelled and optimised the laccase 3D structure, identifying the active substrate binding sites, and the allowed angular conformations to establish the docking coordinates and modelled the ligands. The authors ran analyses taking into account random conformations and the number of interactions, using ABTS (−7.1 kcal mol−1) and DMP (−4.8 kcal mol−1) as controls. Tetracycline (TE) has no interaction with any binding site; however, it had an energy of −5.2 kcal mol−1, probably due to the high density of polar groups unable to interact with the amino acid residues of the enzyme active site. Sulfisoxazole (SXZ) had an energy of −6.8 kcal mol−1; a hydrophobic interaction with the His423, which is in the H-X-H motif at the CuIII copper-binding site, and a minor atomic interaction distance of 7 Å, was detected. This distance between a ligand atom and a receptor atom indicates the relative orientation of the ligands. Small distances (<1.5 Å) represent an atomic overlap generating a major repulsive force, while large distances result in a less effective energy constant [269][128].
On the other hand, the authors calculated the root mean square deviation (RMSD), in this type of analysis, the structural comparison results from the alignment with crystallised structures. The lower the RMSD is, the higher the similarity between the compared molecules. However, this criterion depends on molecule size [260,270][119][129] because values lower than 3.0 Å (typical RMSD values for homologous proteins) indicate high-quality models. In this case, an RMSD = 1.991 was obtained for laccase-Sulfixazole and RMSD = 1.318 for laccase-tetracycline interaction, supporting the ability of the active laccase binding site to interact with these ligands [271][130].
2.2. Ultrasound (Sonolysis) Combined with Enzymatic Degradation for the Degradation of Antibiotics
Sutar and Rathod (2015) worked out the catalytic degradation of Ciprofloxacin (CIP) but using Laccase (Lac, E.C. 1.10.3.2) in a combined process with sonolysis, achieving 51% degradation under specific conditions (0.02% (w/v) enzyme loading; 60 °C; 75 W input power; 22 kHz frequency; 50% duty cycle and 200 rpm agitation); exceeding the degradation obtained in the conventional process (16%) and reducing the treatment time [272][131].
Chakma et al. (2020) investigated the links between enzymatic (Horseradish Peroxidase (HRP, EC: 1.11.1.7) coupled with ultrasound processes for Ciprofloxacin (CIP) degradation and found that ultrasound and cavitation improve the degradation efficiency by increasing the interaction between enzyme and organic molecules (kinetic energy). In this sense, Ciprofloxacin (CIP) is enzymatically transformed, so there is no significant improvement in the sono-enzymatic process at which the highest degradation rates at optimum conditions (pH 7.0 and 25 °C) were 68.41%. In contrast, the intermediates, such as acetic acid, ethyl alcohol and other products with -OH groups generated during the sono-enzymatic reaction are less toxic than those formed in the enzymatic process; this means that ultrasound favours mineralization by degrading the intermediates formed during enzymatic treatment [196][53].
References
- Pan, M.; Chu, L.M. Adsorption and degradation of five selected antibiotics in agricultural soil. Sci. Total Environ. 2016, 545–546, 48–56.
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment-Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338.
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Fels, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481.
- Wu, S.; Hu, Y.H. A comprehensive review on catalysts for electrocatalytic and photoelectrocatalytic degradation of antibiotics. Chem. Eng. J. 2021, 409, 127739.
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Env. Manag. 2011, 92, 2304–2347.
- Abdurahman, M.H.; Abdullah, A.Z.; Shoparwe, N.F. A comprehensive review on sonocatalytic, photocatalytic, and sonophotocatalytic processes for the degradation of antibiotics in water: Synergistic mechanism and degradation pathway. Chem. Eng. J. 2021, 413, 127412.
- Loftin, K.A.; Adams, C.D.; Meyer, M.T.; Surampalli, R. Effects of ionic strength, temperature, and pH on degradation of selected antibiotics. J. Environ. Qual. 2008, 37, 378–386.
- Calcio Gaudino, E.; Canova, E.; Liu, P.; Wu, Z.; Cravotto, G. Degradation of Antibiotics in Wastewater: New Advances in Cavitational Treatments. Molecules 2021, 26, 617.
- Zeghioud, H.; Kamagate, M.; Coulibaly, L.S.; Rtimi, S.; Assadi, A.A. Photocatalytic degradation of binary and ternary mixtures of antibiotics: Reactive species investigation in pilot scale. Chem. Eng. Res. Des. 2019, 144, 300–309.
- Dorival-Garcia, N.; Zafra-Gomez, A.; Navalon, A.; Gonzalez-Lopez, J.; Hontoria, E.; Vilchez, J.L. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions. J. Environ. Manag. 2013, 120, 75–83.
- Lin, K.; Gan, J. Sorption and degradation of wastewater-associated non-steroidal anti-inflammatory drugs and antibiotics in soils. Chemosphere 2011, 83, 240–246.
- Ji, Y.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808.
- Jiang, M.; Wang, L.; Ji, R. Biotic and abiotic degradation of four cephalosporin antibiotics in a lake surface water and sediment. Chemosphere 2010, 80, 1399–1405.
- Braschi, I.; Blasioli, S.; Fellet, C.; Lorenzini, R.; Garelli, A.; Pori, M.; Giacomini, D. Persistence and degradation of new beta-lactam antibiotics in the soil and water environment. Chemosphere 2013, 93, 152–159.
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64.
- Ruan, Y.; Kumar Awasthi, M.; Cai, L.; Lu, H.; Xu, X.; Li, W. Simultaneous aerobic denitrification and antibiotics degradation by strain Marinobacter hydrocarbonoclasticus RAD-2. Bioresour. Technol. 2020, 313, 123609.
- Zhang, Y.; Xu, J.; Zhong, Z.; Guo, C.; Li, L.; He, Y.; Fan, W.; Chen, Y. Degradation of sulfonamides antibiotics in lake water and sediment. Environ. Sci. Pollut. Rese. Int. 2013, 20, 2372–2380.
- Nnenna, F.-P.; Lekiah, P.; Obemeata, O. Degradation of antibiotics by bacteria and fungi from the aquatic environment. J. Toxicol. Environ. Health Sci. 2011, 30, 275–285.
- Alexy, R.; Kumpel, T.; Kummerer, K. Assessment of degradation of 18 antibiotics in the Closed Bottle Test. Chemosphere 2004, 57, 505–512.
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour. Technol. 2013, 131, 476–484.
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233.
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Selective degradation of sulfonamide antibiotics by peroxymonosulfate alone: Direct oxidation and nonradical mechanisms. Chem. Eng. J. 2018, 334, 2539–2546.
- Lange, F.; Cornelissen, S.; Kubac, D.; Sein, M.M.; von Sonntag, J.; Hannich, C.B.; Golloch, A.; Heipieper, H.J.; Moder, M.; von Sonntag, C. Degradation of macrolide antibiotics by ozone: A mechanistic case study with clarithromycin. Chemosphere 2006, 65, 17–23.
- Pelalak, R.; Alizadeh, R.; Ghareshabani, E.; Heidari, Z. Degradation of sulfonamide antibiotics using ozone-based advanced oxidation process: Experimental, modeling, transformation mechanism and DFT study. Sci. Total Environ. 2020, 734, 139446.
- Wu, X.; Zhao, W.; Huang, Y.; Zhang, G. A mechanistic study of amorphous CoSx cages as advanced oxidation catalysts for excellent peroxymonosulfate activation towards antibiotics degradation. Chem. Eng. J. 2020, 381, 122768.
- Guo, W.; Su, S.; Yi, C.; Ma, Z. Degradation of antibiotics amoxicillin by Co3O4-catalyzed peroxymonosulfate system. Environ. Prog. Sustain. Energ. 2013, 32, 193–197.
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coordin. Chem. Rev. 2019, 395, 63–85.
- Rokesh, K.; Sakar, M.; Do, T.O. Emerging Hybrid Nanocomposite Photocatalysts for the Degradation of Antibiotics: Insights into Their Designs and Mechanisms. Nanomaterials 2021, 11, 572.
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023.
- Chen, D.; Li, B.; Pu, Q.; Chen, X.; Wen, G.; Li, Z. Preparation of Ag-AgVO3/g-C3N4 composite photo-catalyst and degradation characteristics of antibiotics. J. Hazard. Mater. 2019, 373, 303–312.
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397.
- He, X.; Mezyk, S.P.; Michael, I.; Fatta-Kassinos, D.; Dionysiou, D.D. Degradation kinetics and mechanism of beta-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. J. Hazard. Mater. 2014, 279, 375–383.
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540.
- Saidulu, D.; Gupta, B.; Gupta, A.K.; Ghosal, P.S. A review on occurrences, eco-toxic effects, and remediation of emerging contaminants from wastewater: Special emphasis on biological treatment based hybrid systems. J. Environ. Chem. Eng. 2021, 9, 105282.
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799.
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52.
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449.
- Zheng, X.; Xu, S.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid. Interface Sci. 2018, 527, 202–213.
- Abellán, M.N.; Giménez, J.; Esplugas, S. Photocatalytic degradation of antibiotics: The case of sulfamethoxazole and trimethoprim. Catal. Today 2009, 144, 131–136.
- Ding, J.; Dai, Z.; Qin, F.; Zhao, H.; Zhao, S.; Chen, R. Z-scheme BiO1-xBr/Bi2O2CO3 photocatalyst with rich oxygen vacancy as electron mediator for highly efficient degradation of antibiotics. Appl. Catal. B Environ. 2017, 205, 281–291.
- Wang, K.; Li, Y.; Zhang, G.; Li, J.; Wu, X. 0D Bi nanodots/2D Bi3NbO7 nanosheets heterojunctions for efficient visible light photocatalytic degradation of antibiotics: Enhanced molecular oxygen activation and mechanism insight. Appl. Catal. B Environ. 2019, 240, 39–49.
- Yan, W.; Yan, L.; Jing, C. Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms. Appl. Catal. B Environ. 2019, 244, 475–485.
- Wang, A.; Zheng, Z.; Wang, H.; Chen, Y.; Luo, C.; Liang, D.; Hu, B.; Qiu, R.; Yan, K. 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl. Catal. B Environ. 2020, 277, 119171.
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Guo, F.; Tang, Y.; Lu, C. Construction of CuBi2O4/Bi2MoO6 p-n heterojunction with nanosheets-on-microrods structure for improved photocatalytic activity towards broad-spectrum antibiotics degradation. Chem. Eng. J. 2020, 394, 125009.
- Li, S.; Wang, C.; Liu, Y.; Xue, B.; Jiang, W.; Liu, Y.; Mo, L.; Chen, X. Photocatalytic degradation of antibiotics using a novel Ag/Ag2S/Bi2MoO6 plasmonic p-n heterojunction photocatalyst: Mineralization activity, degradation pathways and boosted charge separation mechanism. Chem. Eng. J. 2021, 415, 128991.
- Che, H.; Che, G.; Jiang, E.; Liu, C.; Dong, H.; Li, C. A novel Z-Scheme CdS/Bi3O4Cl heterostructure for photocatalytic degradation of antibiotics: Mineralization activity, degradation pathways and mechanism insight. J. Taiwan Inst. Chem. Eng. 2018, 91, 224–234.
- Li, H.; Li, T.; He, S.; Zhou, J.; Wang, T.; Zhu, L. Efficient degradation of antibiotics by non-thermal discharge plasma: Highlight the impacts of molecular structures and degradation pathways. Chem. Eng. J. 2020, 395, 125091.
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Bradu, C.; Parvulescu, V.I. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416.
- Kim, K.-S.; Yang, C.-S.; Mok, Y.S. Degradation of veterinary antibiotics by dielectric barrier discharge plasma. Chem. Eng. J. 2013, 219, 19–27.
- Sarangapani, C.; Ziuzina, D.; Behan, P.; Boehm, D.; Gilmore, B.F.; Cullen, P.J.; Bourke, P. Degradation kinetics of cold plasma-treated antibiotics and their antimicrobial activity. Sci. Rep. 2019, 9, 3955.
- Kong, D.; Liang, B.; Yun, H.; Cheng, H.; Ma, J.; Cui, M.; Wang, A.; Ren, N. Cathodic degradation of antibiotics: Characterization and pathway analysis. Water Res. 2015, 72, 281–292.
- Lin, H.; Zhang, J.; Chen, H.; Wang, J.; Sun, W.; Zhang, X.; Yang, Y.; Wang, Q.; Ma, J. Effect of temperature on sulfonamide antibiotics degradation, and on antibiotic resistance determinants and hosts in animal manures. Sci. Total Environ. 2017, 607–608, 725–732.
- Chakma, S.; Dikshit, P.K.; Galodiya, M.N.; Giri, A.S.; Moholkar, V.S. The role of ultrasound in enzymatic degradation mechanism. J. Taiwan Inst. Chem. Eng. 2020, 107, 54–71.
- Adewuyi, Y.G. Sonochemistry in environmental remediation. 2. Heterogeneous sonophotocatalytic oxidation processes for the treatment of pollutants in water. Environ. Sci. Technol. 2005, 39, 8557–8570.
- De Bel, E.; Dewulf, J.; Witte, B.D.; Van Langenhove, H.; Janssen, C. Influence of pH on the sonolysis of ciprofloxacin: Biodegradability, ecotoxicity and antibiotic activity of its degradation products. Chemosphere 2009, 77, 291–295.
- Liu, P.; Wu, Z.; Abramova, A.V.; Cravotto, G. Sonochemical processes for the degradation of antibiotics in aqueous solutions: A review. Ultrason. Sonochem. 2021, 74, 105566.
- Li, L.; Wei, D.; Wei, G.; Du, Y. Oxidation of cefazolin by potassium permanganate: Transformation products and plausible pathways. Chemosphere 2016, 149, 279–285.
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 2004, 49, 1–14.
- Fan, Y.; Zhou, Z.; Feng, Y.; Zhou, Y.; Wen, L.; Shih, K. Degradation mechanisms of ofloxacin and cefazolin using peroxymonosulfate activated by reduced graphene oxide-CoFe2O4 composites. Chem. Eng. J. 2020, 383, 123056.
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C.; Hassani, A.; Orooji, Y. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manag. 2020, 267, 110629.
- Gurkan, Y.Y.; Turkten, N.; Hatipoglu, A.; Cinar, Z. Photocatalytic degradation of cefazolin over N-doped TiO2 under UV and sunlight irradiation: Prediction of the reaction paths via conceptual DFT. Chem. Eng. J. 2012, 184, 113–124.
- Chen, Y.; Tian, H.; Zhu, W.; Zhang, X.; Li, R.; Chen, C.; Huang, Y. l-Cysteine directing synthesis of BiOBr nanosheets for efficient cefazolin photodegradation: The pivotal role of thiol. J. Hazard. Mater. 2021, 414, 125544.
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020, 381, 120742.
- Liu, J.; Li, Z.; Wang, M.; Jin, C.; Kang, J.; Tang, Y.; Li, S. Eu2O3/Co3O4 nanosheets for levofloxacin removal via peroxymonosulfate activation: Performance, mechanism and degradation pathway. Separat. Pur. Technol. 2021, 274, 118666.
- He, Z.; Zheng, W.; Li, M.; Liu, W.; Zhang, Y.; Wang, Y. Fe2P/biocarbon composite derived from a phosphorus-containing biomass for levofloxacin removal through peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 130928.
- Xu, K.; Ben, W.; Ling, W.; Zhang, Y.; Qu, J.; Qiang, Z. Impact of humic acid on the degradation of levofloxacin by aqueous permanganate: Kinetics and mechanism. Water Res. 2017, 123, 67–74.
- Zhou, Y.; Gao, Y.; Jiang, J.; Shen, Y.M.; Pang, S.Y.; Song, Y.; Guo, Q. A comparison study of levofloxacin degradation by peroxymonosulfate and permanganate: Kinetics, products and effect of quinone group. J. Hazard. Mater. 2021, 403, 123834.
- Liang, J.; Hou, Y.; Zhu, H.; Xiong, J.; Huang, W.; Yu, Z.; Wang, S. Levofloxacin degradation performance and mechanism in the novel electro-Fenton system constructed with vanadium oxide electrodes under neutral pH. Chem. Eng. J. 2022, 433, 133574.
- Zhang, Y.; Hua, S.; Sun, X.; Liu, Z.; Dang, Y.; Zhang, L.; Zhou, Y. A novel electrochemical cathode based on sea urchin-like NiO/Co3O4 composite inducing efficient Fenton-like process for levofloxacin degradation. Appl. Catal. A 2021, 628, 118403.
- Wang, A.; Chen, Z.; Zheng, Z.; Xu, H.; Wang, H.; Hu, K.; Yan, K. Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous microspheres. Chem. Eng. J. 2020, 379, 122340.
- Gong, Y.; Li, J.; Zhang, Y.; Zhang, M.; Tian, X.; Wang, A. Partial degradation of levofloxacin for biodegradability improvement by electro-Fenton process using an activated carbon fiber felt cathode. J. Hazard. Mater. 2016, 304, 320–328.
- Hu, Z.; Ge, M.; Guo, C. Efficient removal of levofloxacin from different water matrices via simultaneous adsorption and photocatalysis using a magnetic Ag3PO4/rGO/CoFe2O4 catalyst. Chemosphere 2021, 268, 128834.
- Rong, F.; Xue, Y.; Tang, W.; Lu, Q.; Wei, M.; Guo, E.; Pang, Y. Visible-light-active 1D Ag-CoWO4/CdWO4 plasmonic photocatalysts boosting levofloxacin conversion. J. Taiwan Inst. Chem. Eng. 2022, 133, 104267.
- Wei, H.; Hu, D.; Su, J.; Li, K. Intensification of levofloxacin sono-degradation in a US/H2O2 system with Fe3O4 magnetic nanoparticles. Chin. J. Chem. Eng. 2015, 23, 296–302.
- Conde-Cid, M.; Fernandez-Calvino, D.; Novoa-Munoz, J.C.; Arias-Estevez, M.; Diaz-Ravina, M.; Fernandez-Sanjurjo, M.J.; Nunez-Delgado, A.; Alvarez-Rodriguez, E. Biotic and abiotic dissipation of tetracyclines using simulated sunlight and in the dark. Sci. Total Environ. 2018, 635, 1520–1529.
- Norzaee, S.; Taghavi, M.; Djahed, B.; Kord Mostafapour, F. Degradation of Penicillin G by heat activated persulfate in aqueous solution. J. Environ. Manag. 2018, 215, 316–323.
- Zhu, L.; Santiago-Schubel, B.; Xiao, H.; Hollert, H.; Kueppers, S. Electrochemical oxidation of fluoroquinolone antibiotics: Mechanism, residual antibacterial activity and toxicity change. Water Res. 2016, 102, 52–62.
- Jiang, Y.; Ran, J.; Mao, K.; Yang, X.; Zhong, L.; Yang, C.; Feng, X.; Zhang, H. Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments. Ecotoxicol. Environ. Saf. 2022, 236, 113464.
- Yang, Y.; Kou, L.; Fan, Q.; Jiang, K.; Wang, J. Simultaneous recovery of phosphate and degradation of antibiotics by waste sludge-derived biochar. Chemosphere 2022, 291, 132832.
- Al-Jubouri, S.M.; Al-Jendeel, H.A.; Rashid, S.A.; Al-Batty, S. Antibiotics adsorption from contaminated water by composites of ZSM-5 zeolite nanocrystals coated carbon. J. Water. Proc. Eng. 2022, 47, 102745.
- Bekkali, C.E.; Bouyarmane, H.; Karbane, M.E.; Masse, S.; Saoiabi, A.; Coradin, T.; Laghzizil, A. Zinc oxide-hydroxyapatite nanocomposite photocatalysts for the degradation of ciprofloxacin and ofloxacin antibiotics. Colloids. Surf. A Physicochem. Eng. Aspects. 2018, 539, 364–370.
- Felis, E.; Buta-Hubeny, M.; Zielinski, W.; Hubeny, J.; Harnisz, M.; Bajkacz, S.; Korzeniewska, E. Solar-light driven photodegradation of antimicrobials, their transformation by-products and antibiotic resistance determinants in treated wastewater. Sci. Total Environ. 2022, 836, 155447.
- Zhang, W.; Liu, Y.; Li, C. Photocatalytic degradation of ofloxacin on Gd2Ti2O7 supported on quartz spheres. J. Phys. Chem. Solids. 2018, 118, 144–149.
- Pal, S.; Ahamed, Z.; Pal, P. Removal of antibiotics and pharmaceutically active compounds from water Environment: Experiments towards industrial scale up. Separat. Purif. Technol. 2022, 295, 121249.
- Reis, A.C.; Kolvenbach, B.A.; Nunes, O.C.; Corvini, P.F.X. Biodegradation of antibiotics: The new resistance determinants—Part I. N Biotechnol. 2020, 54, 34–51.
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent advancement in remediation of synthetic organic antibiotics from environmental matrices: Challenges and perspective. Bioresour. Technol. 2021, 319, 124161.
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264.
- Langbehn, R.K.; Michels, C.; Soares, H.M. Antibiotics in wastewater: From its occurrence to the biological removal by environmentally conscious technologies. Environ. Pollut. 2021, 275, 116603.
- Zhou, W.; Zhang, W.; Cai, Y. Laccase immobilization for water purification: A comprehensive review. Chem. Eng. J. 2021, 403, 126272.
- Becker, D.; Varela Della Giustina, S.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barcelo, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509.
- Bilal, M.; Ashraf, S.S.; Barcelo, D.; Iqbal, H.M.N. Biocatalytic degradation/redefining "removal" fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019, 691, 1190–1211.
- Park, H.; Choung, Y.-K. Degradation of Antibiotics (Tetracycline, Sulfathiazole, Ampicillin) Using Enzymes of Glutathion S-Transferase. Human. Ecol. Risk Assess. Int. J. 2007, 13, 1147–1155.
- Wen, X.; Jia, Y.; Li, J. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from Phanerochaete chrysosporium. J. Hazard. Mater. 2010, 177, 924–928.
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871.
- Tian, Q.; Dou, X.; Huang, L.; Wang, L.; Meng, D.; Zhai, L.; Shen, Y.; You, C.; Guan, Z.; Liao, X. Characterization of a robust cold-adapted and thermostable laccase from Pycnoporus sp. SYBC-L10 with a strong ability for the degradation of tetracycline and oxytetracycline by laccase-mediated oxidation. J. Hazard. Mater. 2020, 382, 121084.
- Copete-Pertuz, L.S.; Plácido, J.; Serna-Galvis, E.A.; Torres-Palma, R.A.; Mora, A. Elimination of Isoxazolyl-Penicillins antibiotics in waters by the ligninolytic native Colombian strain Leptosphaerulina sp. considerations on biodegradation process and antimicrobial activity removal. Sci. Total Environ. 2018, 630, 1195–1204.
- Zhang, C.; You, S.; Zhang, J.; Qi, W.; Su, R.; He, Z. An effective in-situ method for laccase immobilization: Excellent activity, effective antibiotic removal rate and low potential ecological risk for degradation products. Bioresour. Technol. 2020, 308, 123271.
- Sun, X.; Leng, Y.; Wan, D.; Chang, F.; Huang, Y.; Li, Z.; Xiong, W.; Wang, J. Transformation of Tetracycline by Manganese Peroxidase from Phanerochaete chrysosporium. Molecules 2021, 26.
- Weng, S.S.; Ku, K.L.; Lai, H.T. The implication of mediators for enhancement of laccase oxidation of sulfonamide antibiotics. Bioresour. Technol. 2012, 113, 259–264.
- Hakulinen, N.; Rouvinen, J. Three-dimensional structures of laccases. CMLS 2015, 72, 857–868.
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure–function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enz. 2011, 68, 117–128.
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Rodríguez-Vázquez, R.; Delgado-Boada, J.M. Fungal laccases. Fung. Biol. Rev. 2013, 27, 67–82.
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase:microbial sources, production, purification, and potential biotechnological applications. Enz. Res. 2011, 2011, 217861.
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin biodegradation with laccase-mediator systems. Front. Ener. Res. 2014, 2, 12.
- Falade, A.O.; Nwodo, U.U.; Iweriebor, B.C.; Green, E.; Mabinya, L.V.; Okoh, A.I. Lignin peroxidase functionalities and prospective applications. Microbiol. Open 2017, 6, e00394.
- Wesenberg, D.; Kyriakides, I.; Agathos, S.N. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 2003, 22, 161–187.
- Kong, W.; Fu, X.; Wang, L.; Alhujaily, A.; Zhang, J.; Ma, F.; Zhang, X.; Yu, H. A novel and efficient fungal delignification strategy based on versatile peroxidase for lignocellulose bioconversion. Biotechnol. Biofuels. 2017, 10, 218.
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513.
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971.
- Majeau, J.-A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Biores. Technol. 2010, 101, 2331–2350.
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A brief history of colour, the environmental impact of synthetic dyes and removal by using laccases. Molecules 2021, 26, 3813.
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 832.
- Navada, K.K.; Kulal, A. Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int. Biodet. Biodegrad. 2019, 138, 63–69.
- Najafabadipour, N.; Mojtabavi, S.; Jafari-Nodoushan, H.; Samadi, N.; Faramarzi, M.A. High efficiency of osmotically stable laccase for biotransformation and micro-detoxification of levofloxacin in the urea-containing solution: Catalytic performance and mechanism. Colloids. Surf. B Biointerf. 2021, 207, 112022.
- Kelbert, M.; Pereira, C.S.; Daronch, N.A.; Cesca, K.; Michels, C.; de Oliveira, D.; Soares, H.M. Laccase as an efficacious approach to remove anticancer drugs: A study of doxorubicin degradation, kinetic parameters, and toxicity assessment. J. Hazard. Mater. 2021, 409, 124520.
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Poveda-Cuevas, S.A.; Reyes-Guzmán, E.A.; Poutou-Piñales, R.A.; Reyes-Montaño, E.A.; Pedroza-Rodríguez, A.M.; Rodríguez-Vázquez, R.; Cardozo-Bernal, Á.M. Computational analysis and low-scale constitutive expression of laccases synthetic genes GlLCC1 from Ganoderma lucidum and POXA 1B from Pleurotus ostreatus in Pichia pastoris. PLoS ONE 2015, 10, e0116524.
- Sáenz-Suárez, H.; Chávez-Zobbel, A.; Lareo, L.R.; Oribio-Quinto, C.; Martínez-Mendoza, J. Predicción computacional de estructura terciaria de las proteínas humanas Hsp27, aB-cristalina y HspB8. Univ. Sci. 2011, 16, 15–28.
- Zárate-Bonilla, L.J.; del Portillo, P.; Sáenz-Suárez, H.; Janneth, G.-S.; Barreto-Sampaio, G.E.; Poutou-Piñales, R.A.; Felipe Rey, A.; Rey, J.G. Computational modeling and preliminary iroN, fepA, cirA gene expression in Salmonella Enteritidis under iron deficiency induced conditions. Poult. Sci. 2014, 93, 221–230.
- Sáenz-Suárez, H.; Rivera-Hoyos, C.; Morales-Álvarez, E.; Poutou-Piñales, R.; Sáenz-Moreno, J.; Pedroza-Rodríguez, A. Modelación computacional preliminar de la estructura 3D de dos lacasas fúngicas. Salud. Arte. Cuidado. 2014, 7, 5–16.
- Sáenz-Suárez, H.; Poutou-Piñales, R.A.; González-Santos, J.; Barreto, G.E.; Prieto-Navarrera, L.P.; Sáenz-Moreno, J.A.; Landázuri, P.; Barrera-Avellaneda, L.A. Prediction of glycation sites: New insights from protein structural analysis. Turk. J. Biol. 2016, 40, 12–25.
- Niño-Gómez, D.C.; Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Reyes-Montaño, E.A.; Vargas-Alejo, N.E.; Ramírez-Casallas, I.N.; Erkan Turkmen, K.; Sáenz-Suárez, H.; Sáenz-Moreno, J.A.; Poutou-Piñales, R.A.; et al. “In silico” characterization of 3-phytase A and 3-phytase B from Aspergillus niger. Enz. Res. 2017, 2017, 9746191.
- Sáenz, H.; Lareo, L.; Poutou, R.A.; Sosa, C.; Barrera, L.A. Predicción computacional de la estructura terciaria de la iduronato 2-sulfato sulfatasa humana. Biomédica 2007, 27, 7–20.
- Ardila-Leal, L.D.; Monterey-Gutiérrez, P.A.; Poutou-Piñales, R.A.; Quevedo-Hidalgo, B.E.; Galindo, J.F.; Pedroza-Rodríguez, A.M. Recombinant laccase rPOXA 1B real-time and accelerated stability studies supported by molecular dynamics. BMC Biotechnol. 2021, 21, 37.
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102.
- Ardila-Leal, L.D. Producción a escala piloto (10L) y caracterización de un concentrado enzimático de rPOXA 1B para la remoción de colorantes. In Microbiology; Pontificia Universidad Javeriana: Bogotá D.C., Colombia, 2021; p. 361.
- Sarkar, S.; Banerjee, A.; Chakraborty, N.; Soren, K.; Chakraborty, P.; Bandopadhyay, R. Structural-functional analyses of textile dye degrading azoreductase, laccase and peroxidase: A comparative in silico study. Elect. J. Biotechnol. 2020, 43, 48–54.
- Singh, A.; Kumari, S.; Pal, T.K. In silico analysis for laccase-mediated bioremediation of the emerging pharmaceutical pollutants. Int. J. Bioautom. 2015, 19, 423–432.
- Yue, S.-Y. Distance-constrained molecular docking by simulated annealing. Prot. Eng. 1990, 4, 177–184.
- Reva, B.A.; Finkelstein, A.V.; Skolnick, J. What is the probability of a chance prediction of a protein structure with an rmsd of 6 å? Fold Des. 1998, 3, 141–147.
- Cárdenas-Moreno, Y.; Espinosa, L.A.; Vieyto, J.C.; González-Durruthy, M.; del Monte-Martinez, A.; Guerra-Rivera, G.; Sánchez López, M.I. Theoretical study on binding interactions of laccase-enzyme from Ganoderma weberianum with multiples ligand substrates with environmental impact. Ann. Prot. Bioinf. 2019, 3, 001–009.
- Sutar, R.S.; Rathod, V.K. Ultrasound assisted Laccase catalyzed degradation of Ciprofloxacin hydrochloride. J. Ind. Eng. Chem. 2015, 31, 276–282.
More
