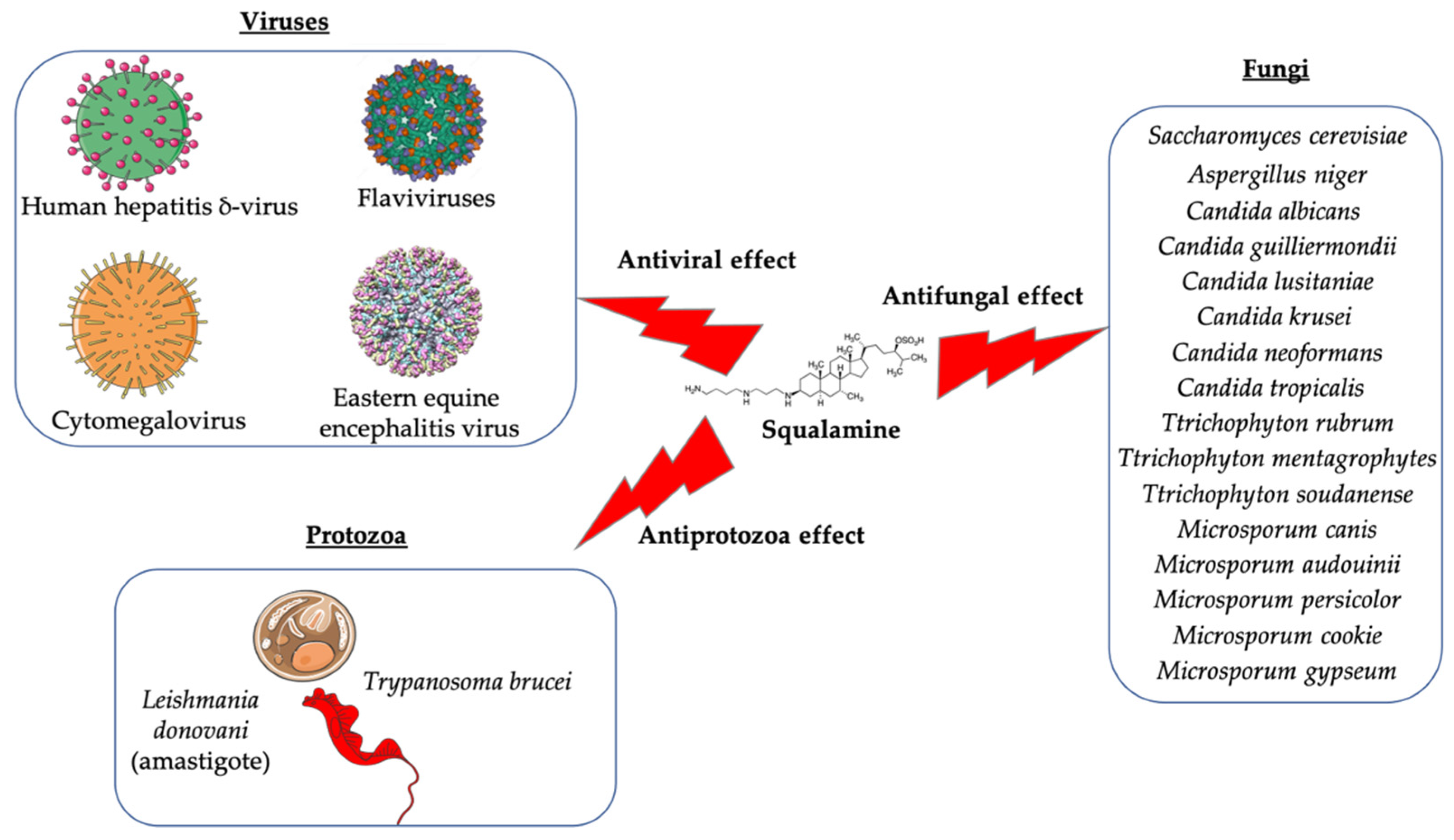
Squalamine is a natural aminosterol that has been discovered in the tissues of the dogfish shark (Squalus acanthias). Studies have previously demonstrated that this promoter compound and its derivatives exhibit potent bactericidal activity against Gram-negative, Gram-positive bacteria, and multidrug-resistant bacteria. The antibacterial activity of squalamine was found to correlate with that of other antibiotics, such as colistin and polymyxins. Still, in the field of microbiology, evidence has shown that squalamine and its derivatives have antifungal activity, antiprotozoa effect against a limited list of protozoa, and could exhibit antiviral activity against both RNA- and DNA-enveloped viruses. Furthermore, squalamine and its derivatives have been identified as being antiangiogenic compounds in the case of several types of cancers and induce a potential positive effect in the case of other diseases such as experimental retinopathy and Parkinson’s disease. Given the diverse effects of the squalamine and its derivatives, in this review we provide the different advances in our understanding of the various effects of these promising molecules and try to draw up a non-exhaustive list of the different mechanisms of actions of squalamine and its derivatives on the human organism and on different pathogens.
- aminosterols
- squalamine
- mechanisms
- antimicrobial
- antiangiogenic
- therapy
1. Introduction
2. Structure of Squalamine and Its Derivatives
| Squalamine and Derivatives | Structure | Pharmacological Activity | References |
|---|---|---|---|
| Squalamine | 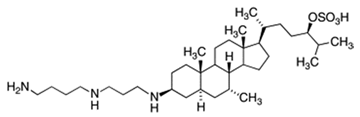 |
Antibacterial activity: Escherichia coli (ATCC 25922, ATCC 54127); Pseudomonas aeruginosa (ATCC 27853, strain PAO1, ATCC 1569, ATCC 15442); Staphylococcus aureus (ATCC 25923, ATCC 6538); Streptococcus pneumoniae (a clinical isolate); Acinetobacter baumannii Antifungal activity: Candida albicans (ATCC 10231, (ATCC 90028); Aspergillus niger (ATCC 16404); Candida glabrata (ATCC 90030); Candida krusei (ATCC 6258); Candida parapsilosis (ATCC 22019) Bloodstream yeast isolates: C. albicans; C. glabrata; C. guilliermondii; C. krusei; C. lusitaniae; C. parapsilosis; C. tropicalis, Cryptococcus neoformans; Trichophyton rubrum; T. mentagrophytes; T. soudanense; Microsporum canis; M. audouinii; M. persicolor; M. cookie; M. gypseum; Tinea capitis Antiviral activity: Dengue virus; Human hepatitis B virus; Human hepatitis δ-virus; Yellow fever virus; Eastern equine encephalitis virus; Murine cytomegalovirus Antiprotozoa activity: Trypanosoma brucei; Leishmania donovani Eucaryote cells: Eukaryote cell (Wehi-231 cells) Antiangiogenic activity: Chicken embryo; MCF-7 (Michigan Cancer Foundation-7); Human breast cancer cell line; Pancreatic (BxPC-3, MiaPaCa-2) and hepatic (HepG2, Huh7) cancer cells; Rat mammary carcinoma and a murine Lewis lung carcinoma; Xenograft models using the chemoresistant MV-522 human non-small cell lung carcinoma and the SD human neuroblastoma lines; PS120/NHE3 fibroblasts cells; Rat brain endothelial (RBE-4) cell; Rabbit VX2 tumor cells Clinical trials for cancer cases: Patients with advanced cancers: Patients with metastasis to the central nervous system; Patients with liver metastasis; Patients with a histologically confirmed diagnosis of nonleukemic malignancy refractory; Patients with advanced solid malignancies; Patients with advanced non-small cell lung cancer Parkinson’s disease: Effect on the gastrointestinal tract (constipation) and neuron motility Retinopathies: Rat choroidal neovascular membrane; Iris neovascularization in cynomolgus monkeys; Ocular neovascularization Clinical trials for Retinopathies: Patients with macular edema |
[5,8,9,11,41,51,52][2][15,17,1118,][1219,]20,21,[13]22,[23,24,14][15][16][17][1825,]26,27,[28,29,19][20][42,3][25][26][27][28][29][30][31][32][33][34][35][36]43,44,][545,][821][22]46,][1030,31,32,33,34,35,36,37,38,39,40,47,]48,49,[50,[23][24[37][38][39][40][41][42][43][44][45][46] |
| Synthesized aminosterol derivatives (ASD) ASD 1 [7-(1,4-diaminobutane)-cholest-5-ene-3β-ol] |
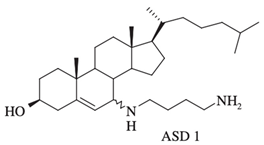 |
Antibacterial activity: E. coli (ATCC 25922); P. aeruginosa (ATCC 27853); S. aureus (ATCC 25923); S. pneumoniae isolates Antifungal: C. albicans (ATCC 90028); C. glabrata (ATCC 90030); C. krusei (ATCC 6258); C. parapsilosis (ATCC 22019) Bloodstream yeast isolates: C. albicans; C. glabrata; C. guilliermondii, C. krusei; C. lusitaniae ; C. parapsilosis; C. tropicali; C. neoformans |
[8,18,20][2][12][14] |
| ASD 2 [7β-(1,4-diaminobutane)-cholestan-3β-ol] | 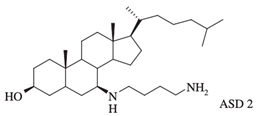 |
Antibacterial activity: E. coli (ATCC 25922); P. aeruginosa (ATCC 27853); S. aureus (ATCC 25923); S. pneumoniae isolates |
[18,32][12][26] |
| 3-amino- and polyaminosterol analogues of squalamine and trodusquemine 4b, 4e, 4n, 4r, 6b, 8b, 8c, 8d, 8e | 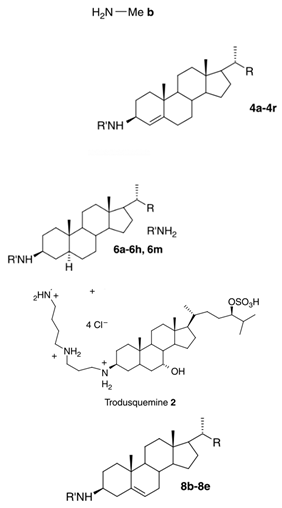 |
Antibacterial activity: E. coli (ATCC 10536); S. aureus (ATCC 6538); Enterococcus faecalis (CIP 103015) Antifungal activity: C. albicans (ATCC 90029); C. tropicalis (CIP 2031); Saccharomyces cerevisiae (ATCC 28383) |
[53][47] |
| 3, 20-amino- and polyaminosteroid analogues of squalamine and trodusquemine |  |
Antibacterial activity: S. aureus; P. aeruginosa; Inquilinus limosus; Burkholderia cepacia |
[54][48] |
| Dimeric sterol-polyamine conjugates (2, 4a, 4b, 5, 6a, 6b, 6c, 7a, 7b) |
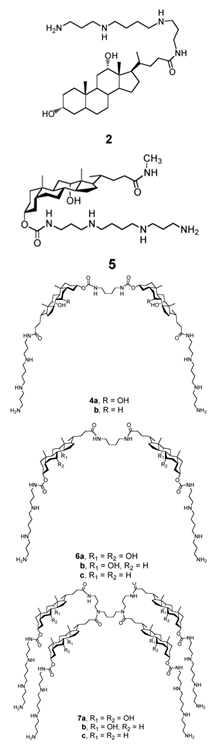 |
Antibacterial activity: E. coli (ATCC 25922 and ESBL clinical isolates); K. pneumoniae (clinical isolates); Acinetobacter spp. (clinical isolates); P. aeruginosa (clinical isolates); group A Streptococcus (clinical isolates); coag. neg. Staphylococcus (clinical isolates); S. aureus (A8115 MSSA, A8816 MRSA, A5948 MRSA 32); Enterococcus faecium (ATCC 29212, ATCC 51299, and clinical isolates) |
[17][10] |
| Squalamine Mimics: Head-to-Tail Dimeric SterolPolyamine Conjugates (1–8) |
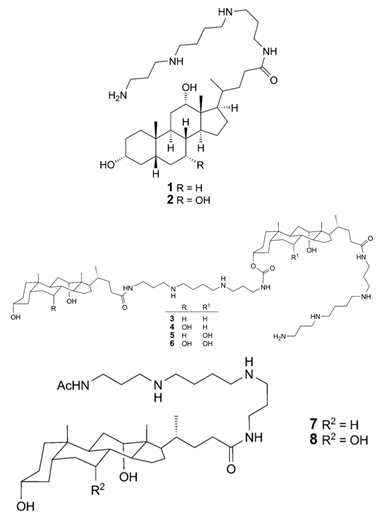 |
Antibacterial activity: E. coli (ATCC 25922 and ESBL clinical isolates); K. pneumoniae (clinical isolates); Acinetobacter spp. (clinical isolates); P. aeruginosa (clinical isolates); E. faecalis (ATCC 29212, ATCC 51299, and clinical isolates); E. faecium (clinical isolates); S. aureus (ATCC 29213, MSSA, and MRSA clinical isolates); Coag. Neg. Staphylococcus (clinical isolates); Group A Streptococcus (clinical isolates) |
[55][49] |
| Squalamine mimics | 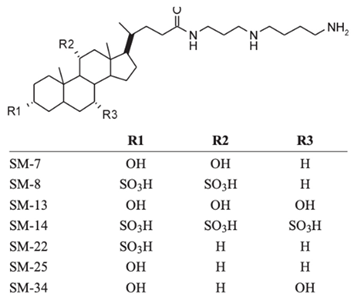 |
Antibacterial activity: E. coli (ATCC 25922); K. pneumoniae (ATCC 13883); P. aeruginosa (ATCC 27853); E. faecalis (ATCC29212); S. aureus (ATCC 25923); S. pyogenes (ATCC 19615); Antifungal activity: C. albicans (ATCC 90028) |
[16][9] |
| Squalamine analogues | 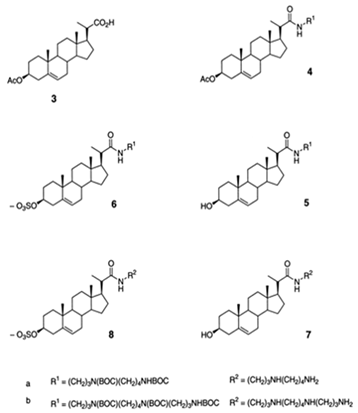 |
Antiprotozoa activity: Leishmania donovani |
[26][20] |
| NV669 | 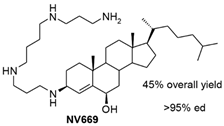 |
Antiangiogenic activity: Pancreatic (BxPC-3, MiaPaCa-2) and hepatic (HepG2, Huh7) cancer cells |
[39][33] |
3. Aminosterols and Their Mode of Action
3.1. Antimicrobial Activity of Squalamine and Its Derivatives
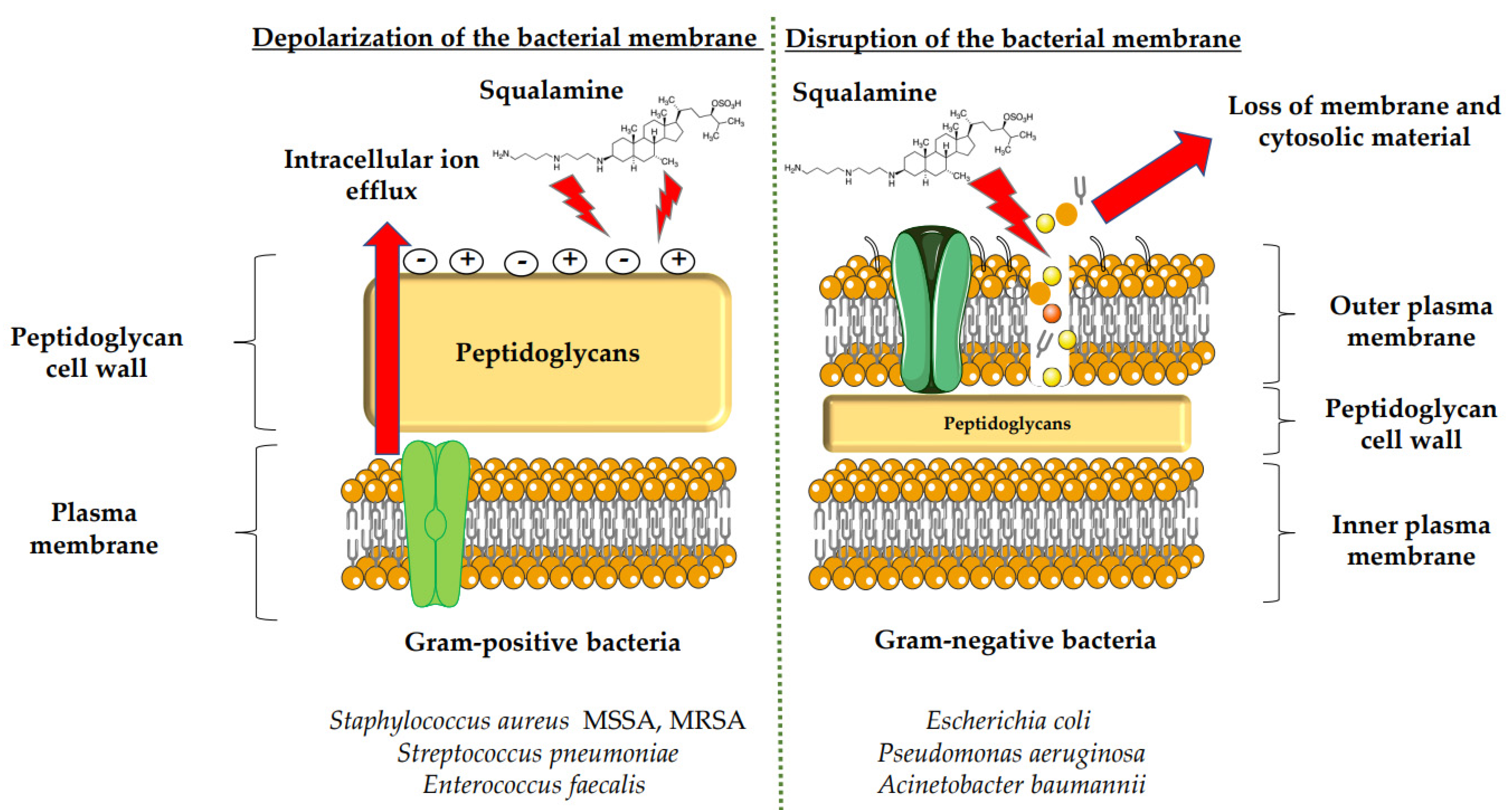
Squalamine displays remarkable efficacy against Gram-negative and Gram-positive bacteria. An analysis of squalamine’s antibacterial efficiency at 250 mg/L was studied against the reference bacterial strains Escherichia coli (E. coli) (American Type Culture Collection (ATCC) 25922), Pseudomonas aeruginosa (P. aeruginosa) (ATCC 27853), Staphylococcus aureus (S. aureus) (ATCC 25923), and a clinical isolate Streptococcus pneumoniae (S. pneumoniae) (recovered from the sputum of a cystic fibrosis patient), and compared to colistin [9][3]. Squalamine exhibited complete killing of P. aeruginosa and E. coli reference strains in 2 h by disrupting membranes, whereas colistin required 4 h. Both compounds react by creating active lesions in the bacterial membrane, leading to emptied bacterial cells. Remarkably, squalamine showed a direct bactericidal effect against the S. aureus reference strain, resulting in a dramatic disruption of the bacterial membrane, with drained cytoplasmic content. Then, the effects of squalamine on bacterial membrane integrity were investigated by measuring intracellular Adenosine triphosphate (ATP) release kinetics for 20 min. It was described that squalamine induced a rapid ATP release from S. aureus and S. pneumoniae that reached 100% of maximal efflux, and depolarization of bacterial membranes was observed [9][3]. These results indicate that squalamine acts by disrupting the outer membranes of Gram-negative bacteria by a detergent-like mechanism of action and by depolarizing the bacterial membranes of Gram-positive bacteria (Figure 1) [9][3]. These squalamine mechanisms of action are preserved even in MDR clinical isolates. MDR bacteria have an ability to overexpress different mechanisms of action of resistance. It that includes drug efflux pumps production, membrane permeability alteration, and enzymatic barrier activation. All these mechanisms of action are well-known to induce a high level of resistance towards quinolones, ß-lactams, and phenicols [13,17,18][7][10][12]. The antibacterial activity of squalamine and synthesized aminosterol derivatives (ASD 1 [7-(1,4-diaminobutane)-cholest-5-ene-3β-ol] and ASD 2 [7β-(1,4-diaminobutane)-cholestan-3β-ol) (Table 1) was investigated for the first time against a large range of MDR bacteria involved in clinical pathologies like cystic fibrosis (CF). Interestingly, antibacterial experimentations revealed minimal inhibitory concentrations (MICs) for E. coli (ATCC 25922) of 2 mg/L for squalamine and 4 mg/L for both ASD 1 and ASD 2. MICs were 8 mg/L for squalamine, 4 mg/L and 8 mg/L for ASD 1 and ASD 2, respectively, for P. aeruginosa (ATCC 27853) strain. MICs were 2 mg/L for squalamine, 2 mg/L and 4 mg/L for ASD 1 and ASD 2, respectively, for S. aureus (ATCC 25923) strain. ASDs were also active against S. pneumoniae isolates with a MIC of 32 mg/L, and against others Gram-positive isolates, with MICs ranging from 0.5 to 8 mg/L. Some studies have suggested that there is a similarity of certain mechanisms of action of ASDs and polymyxins, which suggests that the antibacterial activity of ASDs against Gram-positive bacteria is reduced (Figure 1) [19][13]. The studies of the antimicrobial effects of squalamine have been extended from the experimental phase to the preclinical and clinical phase. Squalamine has been tested as a therapeutic agent to fight against the colonization of the skin by S. aureus. Cutaneous colonization was studied in a mouse model infected by methicillin-susceptible S. aureus (ATCC 25923) and methicillin-resistant S. aureus (MRSA) (Collection de Souches de l’Unité des Rickettsies (CSUR) P102) at 106–108 colony-forming unit/mL (cfu/mL), separately. Squalamine revealed a high degree of viable S. aureus load reduction with bactericidal effect up to 4-log10, during a 2-day experiment. A single dose of squalamine formulated at 1% in a petrolatum-based cream completely eradicated S. aureus (Table 1) [24][18]. Antidermatophyte effect of squalamine and of a synthetic aminosterol derivative was also investigated and showed significant in vitro activity against Trichophyton rubrum (T. rubrum), Trichophyton mentagrophytes (T. mentagrophyte), Trichophyton soudanense (T. soudanense), Microsporum canis (M. canis), Microsporum audouinii (M. audouinii), Microsporum persicolor (M. persicolor), Microsporum cookie (M. cookie), and Microsporum gypseum (M. gypseum), with MICs ranging from 4–16 mg/L for squalamine, and from 2–8 mg/L for the synthetic aminosterol derivative (Table 1) (Figure 2) [25][19]. These findings supported further clinical studies of aminosterols activity against dermatophyte infections, introduced by a double-blinded randomized placebo-controlled clinical trial focused on squalamine effect on tinea capitis. This pathology represents a common fungal infection of the scalp, hair follicles, and hair shafts [57][50]. Clinical trial phase II study shows a statistically significant higher hairgrowth score within the tinea capitis lesions treated with 1% squalamine ointment. In addition, squalamine showed a favorable safety profile when administered topically, as no clinical or biological adverse events were recorded in treated patients. In this clinical trial, squalamine ointment application was well tolerated and showed an encouraging partial clinical activity for the treatment of tinea (Table 1) [58][51].3.2. Antiangiogenesis and Antitumor Activity of Squalamine and Its Derivatives
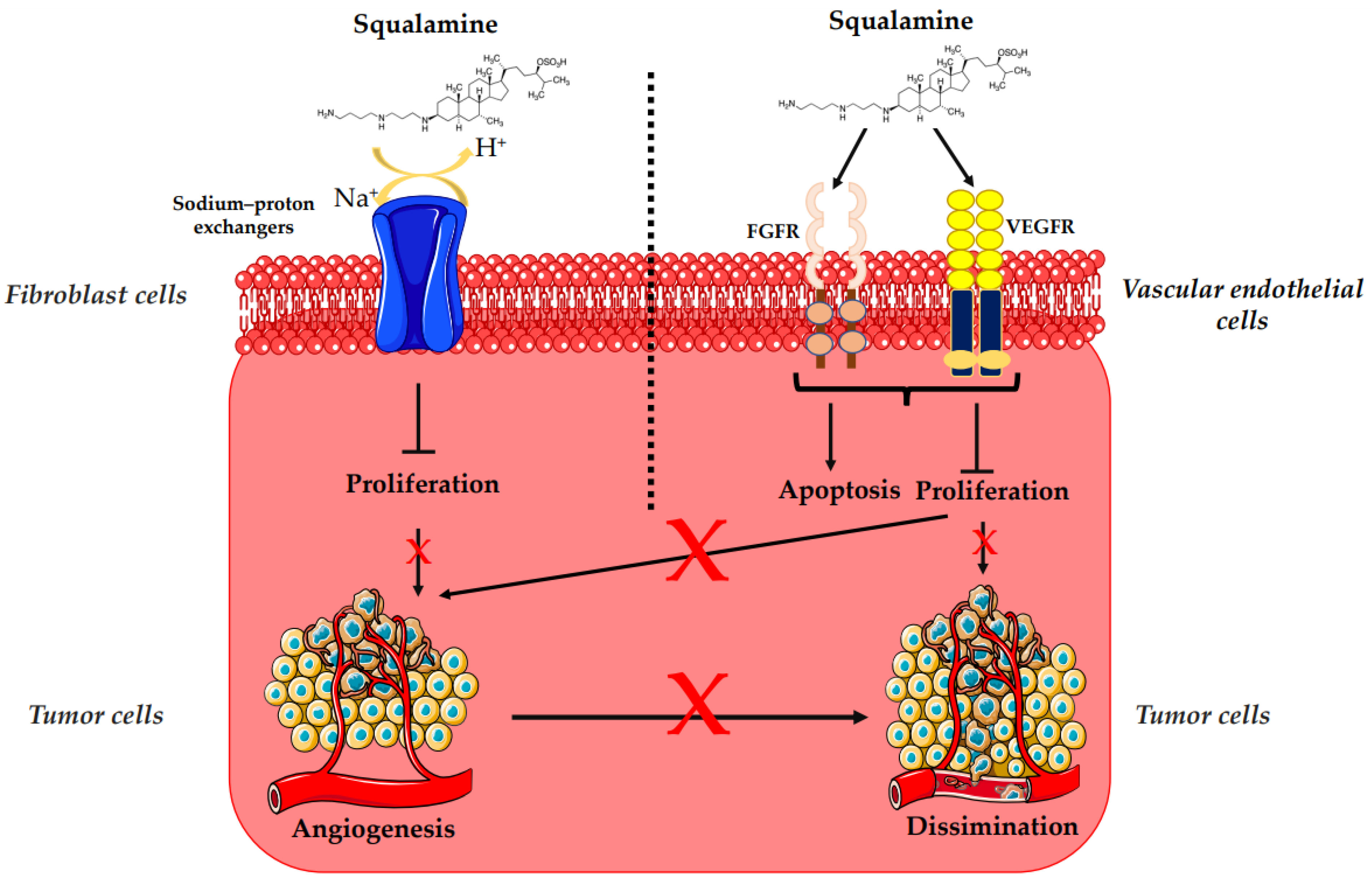
The overexpression of HER-2/neu proto-oncogene is one of the risk factors for the occurrence of breast cancer, which is a major cause of mortality among women worldwide [47][41]. HER-2 receptor-mediated signaling is also known to enhance secretion of vascular endothelial growth factor (VEGF), eliciting increased tumor-associated angiogenesis, which is critical for tumor growth and progression (Figure 3) [48][42]. Regarding treatment, randomized clinical trials based on bevacizumab (a recombinant humanized monoclonal antibody directed against the vascular endothelial growth factor) and trastuzumab (a monoclonal anti-human epidermal growth factor receptor 2 protein antibody) have not demonstrated any additional overall survival benefit [49,50,51,53,54,60][43][44][45][47][48][52]. In that context, squalamine has been used as an alternative antiangiogenic treatment [49,50,51][43][44][45]. Thus, the effects of squalamine on angiogenesis, mitogen-induced proliferation and vascularization, have been studied on a chicken embryo. It was observed that squalamine reduces the vascularization of the tumor; it decreases the levels of the fibroblast growth factor (FGF) and the levels of VEGF. Furthermore, squalamine leads to a decrease in gliomas (brain tumors), cysts (formation of abnormal epithelium), and hemangioblastomas (tumors of vascular origin often in the central nervous system) (Table 1) (Figure 3) [27][21]. The biological mechanism of the antiangiogenic and antitumor effects of squalamine was also evaluated in vitro with MCF-7 (Michigan Cancer Foundation-7) human breast cancer cell line and in vivo with HER-2 breast tumor xenografts, where squalamine treatment (at 2 mg/kg daily) reduced the growth of tumors as compared to controls (Table 1). Thus, to determine the effect of squalamine on the activation of VEGF, human umbilical vein endothelial cells (HUVECs) were grown in vitro in the presence of 50 ng/mL of VEGF, 3.2 μM of squalamine and combinations of VEGF, and different concentrations of squalamine. Significant inhibition of VEGF-induced vascular endothelial cell proliferation by squalamine was revealed. This growth-suppressive effect of squalamine was dose-dependent, with maximal suppression at 3.2 μM. Thus, the antiangiogenic activity of squalamine appears to be mediated by a direct effect on vascular endothelial cells (Figure 3) [5][11].3.3. Potential Effects of Squalamine on Neurodegenerative Diseases and Other Pathologies
3.3.1. Parkinson’s Disease
In animal models and in the context of intestinal pathologies related to neurodegenerative diseases, squalamine and its derivative trodusquemine could have a promotive effect on neurodegenerative disease, especially in cases of Parkinson’s and Alzheimer diseases [52][46]. Squalamine has been explored as a potential agent which has an effect on the gastrointestinal tract, particularly in cases with Parkinson’s disease [35][29].3.3.2. Retinopathies and Ocular Neovascularization
Given that the antiangiogenic effect of squalamine in tumors has already been demonstrated in various studies, there has been a growing interest in evaluating the effect of this aminosterol in the case of retinopathies [37,38,61,62][31][32][53][54]. Indeed, mice with oxygen-induced retinopathy treated subcutaneously with squalamine at 25 mg/kg for 5 days showed improvement in their retinopathy, characterized by a decrease in the number of neovascular nuclei extending beyond the internal limiting membrane on retinal sections. Additionally, in a rat model, systemically administered squalamine lactate partially reduced the development of the choroidal neovascular membrane induced by laser trauma in this animal model (Table 1) [36][30]. In an experimental model of iris neovascularization in cynomolgus monkeys, treated with squalamine 1 mg/kg administered by systemic injection, the aminosterol induced regression of iris neovascularization in all experiments and led to complete regression of iris rubeosis for some of the animals. While the controls presented an intense and persistent neovascularization of the iris, systemic squalamine injection inhibited the development of iris neovascularization and caused partial regression of new vessels in a primate model (Table 1) [37][31]. These encouraging results show that squalamine could be a novel agent for an effective treatment of ocular neovascularization and used as a therapeutic agent for macular degeneration or for retinopathies [37,38,61,62][31][32][53][54].References
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M.; Forrest, J.N.; McCrimmon, D.; Zasloff, M. Squalamine: An Aminosterol Antibiotic from the Shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358.
- Alhanout, K.; Brunel, J.M.; Ranque, S.; Rolain, J.M. In Vitro Antifungal Activity of Aminosterols against Moulds Isolated from Cystic Fibrosis Patients. J. Antimicrob. Chemother. 2010, 65, 1307–1309.
- Alhanout, K.; Malesinki, S.; Vidal, N.; Peyrot, V.; Rolain, J.M.; Brunel, J.M. New Insights into the Antibacterial Mechanism of Action of Squalamine. J. Antimicrob. Chemother. 2010, 65, 1688–1693.
- Rao, M.N.; Shinnar, A.E.; Noecker, L.A.; Chao, T.L.; Feibush, B.; Snyder, B.; Sharkansky, I.; Sarkahian, A.; Zhang, X.; Jones, S.R.; et al. Aminosterols from the Dogfish Shark Squalus Acanthias. J. Nat. Prod. 2000, 63, 631–635.
- Zasloff, M.; Adams, A.P.; Beckerman, B.; Campbell, A.; Han, Z.; Luijten, E.; Meza, I.; Julander, J.; Mishra, A.; Qu, W.; et al. Squalamine as a Broad-Spectrum Systemic Antiviral Agent with Therapeutic Potential. Proc. Natl. Acad. Sci. USA 2011, 108, 15978–15983.
- Li, D.; Williams, J.I.; Pietras, R.J. Squalamine and Cisplatin Block Angiogenesis and Growth of Human Ovarian Cancer Cells with or without HER-2 Gene Overexpression. Oncogene 2002, 21, 2805–2814.
- Salmi, C.; Loncle, C.; Vidal, N.; Letourneux, Y.; Fantini, J.; Maresca, M.; Taïeb, N.; Pagès, J.-M.; Brunel, J.M. Squalamine: An Appropriate Strategy against the Emergence of Multidrug Resistant Gram-Negative Bacteria? PLoS ONE 2008, 3, e2765.
- PubChem Squalamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/72495 (accessed on 6 January 2022).
- Savage, P.B.; Li, C. Cholic Acid Derivatives: Novel Antimicrobials. Expert Opin. Investig. Drugs 2005, 9, 263–272.
- Chen, W.-H.; Shao, X.-B.; Moellering, R.; Wennersten, C.; Regen, S.L. A Bioconjugate Approach toward Squalamine Mimics: Insight into the Mechanism of Biological Action. Bioconjugate Chem. 2006, 17, 1582–1591.
- Márquez-Garbán, D.C.; Gorrín-Rivas, M.; Chen, H.-W.; Sterling, C.; Elashoff, D.; Hamilton, N.; Pietras, R.J. Squalamine Blocks Tumor-Associated Angiogenesis and Growth of Human Breast Cancer Cells with or without HER-2/Neu Overexpression. Cancer Lett. 2019, 449, 66–75.
- Lavigne, J.-P.; Brunel, J.-M.; Chevalier, J.; Pagès, J.-M. Squalamine, an Original Chemosensitizer to Combat Antibiotic-Resistant Gram-Negative Bacteria. J. Antimicrob. Chemother. 2010, 65, 799–801.
- Alhanout, K.; Brunel, J.-M.; Raoult, D.; Rolain, J.-M. In Vitro Antibacterial Activity of Aminosterols against Multidrug-Resistant Bacteria from Patients with Cystic Fibrosis. J. Antimicrob. Chemother. 2009, 64, 810–814.
- Djouhri-Bouktab, L.; Alhanout, K.; Andrieu, V.; Stremler, N.; Dubus, J.C.; Raoult, D.; Rolain, J.M.; Brunel, J.M. Soluble Squalamine Tablets for the Rapid Disinfection of Home Nebulizers of Cystic Fibrosis Patients. J. Cyst. Fibros. 2012, 11, 555–559.
- Alhanout, K.; Djouhri, L.; Vidal, N.; Brunel, J.M.; Piarroux, R.; Ranque, S. In Vitro Activity of Aminosterols against Yeasts Involved in Blood Stream Infections. Med. Mycol. 2011, 49, 121–125.
- Hraiech, S.; Brégeon, F.; Brunel, J.-M.; Rolain, J.-M.; Lepidi, H.; Andrieu, V.; Raoult, D.; Papazian, L.; Roch, A. Antibacterial Efficacy of Inhaled Squalamine in a Rat Model of Chronic Pseudomonas Aeruginosa Pneumonia. J. Antimicrob. Chemother. 2012, 67, 2452–2458.
- Nicol, M.; Mlouka, M.A.B.; Berthe, T.; Di Martino, P.; Jouenne, T.; Brunel, J.-M.; Dé, E. Anti-Persister Activity of Squalamine against Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2019, 53, 337–342.
- Djouhri-Bouktab, L.; Alhanout, K.; Andrieu, V.; Raoult, D.; Rolain, J.M.; Brunel, J.M. Squalamine Ointment for Staphylococcus aureus Skin Decolonization in a Mouse Model. J. Antimicrob. Chemother. 2011, 66, 1306–1310.
- Coulibaly, O.; Alhanout, K.; L’Ollivier, C.; Brunel, J.-M.; Thera, M.A.; Djimdé, A.A.; Doumbo, O.K.; Piarroux, R.; Ranque, S. In Vitro Activity of Aminosterols against Dermatophytes. Med. Mycol. 2013, 51, 309–312.
- Khabnadideh, S.; Tan, C.L.; Croft, S.L.; Kendrick, H.; Yardley, V.; Gilbert, I.H. Squalamine Analogues as Potential Anti-Trypanosomal and Anti-Leishmanial Compounds. Bioorg. Med. Chem. Lett. 2000, 10, 1237–1239.
- Sills, A.K.; Williams, J.I.; Tyler, B.M.; Epstein, D.S.; Sipos, E.P.; Davis, J.D.; McLane, M.P.; Pitchford, S.; Cheshire, K.; Gannon, F.H.; et al. Squalamine Inhibits Angiogenesis and Solid Tumor Growth in Vivo and Perturbs Embryonic Vasculature. Cancer Res. 1998, 58, 2784–2792.
- Teicher, B.A.; Williams, J.I.; Takeuchi, H.; Ara, G.; Herbst, R.S.; Buxton, D. Potential of the Aminosterol, Squalamine in Combination Therapy in the Rat 13,762 Mammary Carcinoma and the Murine Lewis Lung Carcinoma. Anticancer Res. 1998, 18, 2567–2573.
- Bhargava, P.; Marshall, J.L.; Dahut, W.; Rizvi, N.; Trocky, N.; Williams, J.I.; Hait, H.; Song, S.; Holroyd, K.J.; Hawkins, M.J. A Phase I and Pharmacokinetic Study of Squalamine, a Novel Antiangiogenic Agent, in Patients with Advanced Cancers. Clin. Cancer Res. 2001, 7, 3912–3919.
- Hao, D.; Hammond, L.A.; Eckhardt, S.G.; Patnaik, A.; Takimoto, C.H.; Schwartz, G.H.; Goetz, A.D.; Tolcher, A.W.; McCreery, H.A.; Mamun, K.; et al. A Phase I and Pharmacokinetic Study of Squalamine, an Aminosterol Angiogenesis Inhibitor. Clin. Cancer Res. 2003, 9, 2465–2471.
- Herbst, R.S.; Hammond, L.A.; Carbone, D.P.; Tran, H.T.; Holroyd, K.J.; Desai, A.; Williams, J.I.; Bekele, B.N.; Hait, H.; Allgood, V.; et al. A Phase I/IIA Trial of Continuous Five-Day Infusion of Squalamine Lactate (MSI-1256F) Plus Carboplatin and Paclitaxel in Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2003, 9, 4108–4115.
- Williams, J.I.; Weitman, S.; Gonzalez, C.M.; Jundt, C.H.; Marty, J.; Stringer, S.D.; Holroyd, K.J.; Mclane, M.P.; Chen, Q.; Zasloff, M.; et al. Squalamine Treatment of Human Tumors in Nu/Nu Mice Enhances Platinum-Based Chemotherapies. Clin. Cancer Res. 2001, 7, 724–733.
- Akhter, S.; Nath, S.K.; Tse, C.M.; Williams, J.; Zasloff, M.; Donowitz, M. Squalamine, a Novel Cationic Steroid, Specifically Inhibits the Brush-Border Na+/H+ Exchanger Isoform NHE3. Am. J. Physiol. 1999, 276, C136–C144.
- Pietras, R.J.; Weinberg, O.K. Antiangiogenic Steroids in Human Cancer Therapy. Evid. Based Complement. Alternat. Med. 2005, 2, 49–57.
- West, C.L.; Mao, Y.-K.; Delungahawatta, T.; Amin, J.Y.; Farhin, S.; McQuade, R.M.; Diwakarla, S.; Pustovit, R.; Stanisz, A.M.; Bienenstock, J.; et al. Squalamine Restores the Function of the Enteric Nervous System in Mouse Models of Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 1477–1491.
- Ciulla, T.A.; Criswell, M.H.; Danis, R.P.; Williams, J.I.; McLane, M.P.; Holroyd, K.J. Squalamine Lactate Reduces Choroidal Neovascularization in a Laser-Injury Model in the Rat. Retina 2003, 23, 808–814.
- Genaidy, M.; Kazi, A.A.; Peyman, G.A.; Passos-Machado, E.; Farahat, H.G.; Williams, J.I.; Holroyd, K.J.; Blake, D.A. Effect of Squalamine on Iris Neovascularization in Monkeys. Retina 2002, 22, 772–778.
- Higgins, R.D.; Sanders, R.J.; Yan, Y.; Zasloff, M.; Williams, J.I. Squalamine Improves Retinal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1507–1512.
- Carmona, S.; Brunel, J.-M.; Bonier, R.; Sbarra, V.; Robert, S.; Borentain, P.; Lombardo, D.; Mas, E.; Gerolami, R. A Squalamine Derivative, NV669, as a Novel PTP1B Inhibitor: In Vitro and In Vivo Effects on Pancreatic and Hepatic Tumor Growth. Oncotarget 2019, 10, 6651–6667.
- Gallo, S.W.; Donamore, B.K.; Pagnussatti, V.E.; Ferreira, C.A.S.; de Oliveira, S.D. Effects of Meropenem Exposure in Persister Cells of Acinetobacter Calcoaceticus-Baumannii. Future Microbiol. 2017, 12, 131–140.
- Chung, E.S.; Wi, Y.M.; Ko, K.S. Variation in Formation of Persister Cells against Colistin in Acinetobacter Baumannii Isolates and Its Relationship with Treatment Failure. J. Antimicrob. Chemother. 2017, 72, 2133–2135.
- Bhargava, N.; Sharma, P.; Capalash, N. Pyocyanin Stimulates Quorum Sensing-Mediated Tolerance to Oxidative Stress and Increases Persister Cell Populations in Acinetobacter baumannii. Infect. Immun. 2014, 82, 3417–3425.
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An Overview of Their Antibacterial, Antibiotic-Enhancing and Antivirulence Activities. Int. J. Antimicrob. Agents 2014, 44, 377–386.
- Tsubery, H.; Ofek, I.; Cohen, S.; Eisenstein, M.; Fridkin, M. Modulation of the Hydrophobic Domain of Polymyxin B Nonapeptide: Effect on Outer-Membrane Permeabilization and Lipopolysaccharide Neutralization. Mol. Pharmacol. 2002, 62, 1036–1042.
- Clausell, A.; Rabanal, F.; Garcia-Subirats, M.; Asunción Alsina, M.; Cajal, Y. Synthesis and Membrane Action of Polymyxin B Analogues. Luminescence 2005, 20, 117–123.
- Di Pasquale, E.; Salmi-Smail, C.; Brunel, J.-M.; Sanchez, P.; Fantini, J.; Maresca, M. Biophysical Studies of the Interaction of Squalamine and Other Cationic Amphiphilic Molecules with Bacterial and Eukaryotic Membranes: Importance of the Distribution Coefficient in Membrane Selectivity. Chem. Phys. Lipids 2010, 163, 131–140.
- Kaptain, S.; Tan, L.K.; Chen, B. Her-2/Neu and Breast Cancer. Diagn. Mol. Pathol. 2001, 10, 139–152.
- Dore-Savard, L.; Lee, E.; Kakkad, S.; Popel, A.S.; Bhujwalla, Z.M. The Angiogenic Secretome in VEGF Overexpressing Breast Cancer Xenografts. Sci. Rep. 2016, 6, 39460.
- Hoffmann-La Roche. A Randomized, Open-Label Study to Compare the Effect of First-Line Treatment with Avastin in Combination with Herceptin/Docetaxel and Herceptin/Docetaxel Alone on Progression-Free Survival in Patients with HER2 Positive Locally Recurrent or Metastatic Breast Cancer. 2015. Available online: clinicaltrials.gov (accessed on 16 September 2021).
- Gianni, L.; Romieu, G.H.; Lichinitser, M.; Serrano, S.V.; Mansutti, M.; Pivot, X.; Mariani, P.; Andre, F.; Chan, A.; Lipatov, O.; et al. AVEREL: A Randomized Phase III Trial Evaluating Bevacizumab in Combination with Docetaxel and Trastuzumab as First-Line Therapy for HER2-Positive Locally Recurrent/Metastatic Breast Cancer. J. Clin. Oncol. 2013, 31, 1719–1725.
- Zhao, M.; Pan, X.; Layman, R.; Lustberg, M.B.; Mrozek, E.; Macrae, E.R.; Wesolowski, R.; Carothers, S.; Puhalla, S.; Shapiro, C.L.; et al. A Phase II Study of Bevacizumab in Combination with Trastuzumab and Docetaxel in HER2 Positive Metastatic Breast Cancer. Investig. New Drugs 2014, 32, 1285–1294.
- Limbocker, R.; Errico, S.; Barbut, D.; Knowles, T.P.J.; Vendruscolo, M.; Chiti, F.; Zasloff, M. Squalamine and Trodusquemine: Two Natural Products for Neurodegenerative Diseases, from Physical Chemistry to the Clinic. Nat. Prod. Rep. 2022, 39, 742–753.
- Salmi, C.; Loncle, C.; Vidal, N.; Laget, M.; Letourneux, Y.; Michel Brunel, J. Antimicrobial Activities of 3-Amino- and Polyaminosterol Analogues of Squalamine and Trodusquemine. J. Enzyme Inhib. Med. Chem. 2008, 23, 860–865.
- Djouhri-Bouktab, L.; Vidal, N.; Rolain, J.M.; Brunel, J.M. Synthesis of New 3,20-Bispolyaminosteroid Squalamine Analogues and Evaluation of Their Antimicrobial Activities. J. Med. Chem. 2011, 54, 7417–7421.
- Chen, W.-H.; Wennersten, C.; Moellering, R.C.; Regen, S.L. Towards Squalamine Mimics: Synthesis and Antibacterial Activities of Head-to-Tail Dimeric Sterol-Polyamine Conjugates. Chem. Biodivers. 2013, 10, 385–393.
- Möhrenschlager, M.; Seidl, H.P.; Ring, J.; Abeck, D. Pediatric Tinea Capitis: Recognition and Management. Am. J. Clin. Dermatol. 2005, 6, 203–213.
- Coulibaly, O.; Thera, M.A.; Koné, A.K.; Siaka, G.; Traoré, P.; Djimdé, A.A.; Brunel, J.-M.; Gaudart, J.; Piarroux, R.; Doumbo, O.K.; et al. A Double-Blind Randomized Placebo-Controlled Clinical Trial of Squalamine Ointment for Tinea Capitis Treatment. Mycopathologia 2015, 179, 187–193.
- Wroblewski, J.J.; Hu, A.Y. Topical Squalamine 0.2% and Intravitreal Ranibizumab 0.5 Mg as Combination Therapy for Macular Edema Due to Branch and Central Retinal Vein Occlusion: An Open-Label, Randomized Study. Ophthalmic Surg. Lasers Imaging Retina 2016, 47, 914–923.
- Connolly, B.; Desai, A.; Garcia, C.A.; Thomas, E.; Gast, M.J. Squalamine Lactate for Exudative Age-Related Macular Degeneration. Ophthalmol. Clin. N. Am. 2006, 19, 381–391, vi.
- Hussain, R.M.; Ciulla, T.A. Emerging Vascular Endothelial Growth Factor Antagonists to Treat Neovascular Age-Related Macular Degeneration. Expert Opin. Emerg. Drugs 2017, 22, 235–246.
