Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Caterina Sagnelli and Version 2 by Vivi Li.
Cardiovascular disease is the most important cause of death worldwide in recent years; an increasing trend is also shown in organ transplant patients subjected to immunosuppressive therapies, in which cardiovascular diseases represent one of the most frequent causes of long-term mortality. This is also linked to immunosuppressant-induced dyslipidemia, which occurs in 27 to 71% of organ transplant recipients.
- cardiovascular disease
- dyslipidemia
- immunosuppressive therapy
- organ transplant
1. Introduction
Atherosclerosis is defined as the accumulation of fatty and fibrous material in the intima layer of an artery, inducing formation of atheroma (i.e., atherosclerotic plaque). Over time, plaque continues to grow, increasing its calcium and fibrous material content, and it can lead to tissue ischemia by obstructing the lumen of the vessel or disrupting itself by occluding the lumen of a distal vessel [1]. Depending on the affected artery, atherosclerotic cardiovascular disease (ASCVD) can cause acute coronary syndromes (ACS), ischemic stroke or transient cerebral ischemic attacks (TIA), and peripheral artery disease (PAD).
In 2019, there were an estimated 523 million cases of cardiovascular diseases (CVD), causing 18.5 million deaths [2]. Moreover, CVDs are a relevant cause of disability, bringing about 194 million and 143 million disability-adjusted life years (DALYs) for ischemic heart disease and stroke, respectively [2].
Dyslipidemia, that is, an alteration in lipid metabolism, is a well-known risk factor for ASCVD development; in particular, low-density lipoprotein cholesterol (LDL) circulating levels are unequivocally established as the principal determinant of atherosclerotic plaque formation and progression [3].
Atherosclerosis is a long process, beginning in the first decades of life, even in people without specific genetic characteristics, regardless of the presence of symptoms [4].
In addition to lipids, other risk factors have been identified in atherosclerosis, such as hyperglycemia, hypertension, tobacco use, and visceral adiposity [5][6][7][8][5,6,7,8].
Moreover, chronic kidney disease (CKD) is a well-known independent risk factor for atherosclerosis, and hemodialysis patients have a higher inflammatory status and more severe impaired blood flow [9].
All the above-mentioned factors contribute to plaque formation, triggering activation of inflammatory pathways, with consequent accumulation of fibrous material and plaque growth [1].
In patients who underwent solid organ transplantation and who were subjected to immunosuppressive therapies, dyslipidemia is very common; consequently, CVDs represent a frequent cause of long-term mortality in these patients, being estimated as the first cause of death in heart and kidney transplant recipients [10][11][10,11], and the second in liver transplant recipients [12].
Particularly in heart transplant recipients, atherosclerosis seems to be more aggressive, increasing the risk of vasculopathy progressively every 5 years, with an additional risk of 10% every 2 years after the transplant [13]. In addition, in these patients a particular form of coronary atherosclerosis was found, named cardiac allograft vasculopathy (CAV), which is morphologically different from typical atheromatous plaque [14].
Several risk factors are involved in the pathogenesis of the atherosclerotic process in these patients, such as transplant rejection, hypertension, dyslipidemia, and diabetes; however, hypercholesterolemia and hypertriglyceridemia are the most frequent metabolism abnormalities in clinical practice [15]. Immunosuppressor-mediated hyperlipidemia is characterized by an increase in LDL cholesterol, VLDL cholesterol, and/or an increase in total plasma triglycerides, mainly VLDL triglycerides [16][17][16,17].
2. Role of Dyslipidemia in Atherosclerosis
Dyslipidemia is defined as an abnormal concentration of lipids in the blood, and it can be present despite normal total cholesterol levels if there is an increase of lipoproteins that carry the cardiovascular risk factor. Lipoproteins are constituted of lipids (such as cholesterol and triglycerides) and proteins called apolipoproteins. Different lipoproteins are distinguished by size, lipid content, and type of apolipoprotein. Low-density lipoproteins (LDL) are small molecules, rich in ApoB-100 apolipoprotein, and are unequivocally correlated with ASCVD [3]. Very low-density lipoproteins (VLDL) and their remnants are rich in triglycerides and are also associated with ASCVD; however, this association seems to be related to the blood concentration of ApoB-containing particles rather than the concentration of triglycerides itself [18]. High-density lipoproteins (HDL) are the smallest lipoproteins and are abundant in apolipoprotein ApoA-I and ApoA-II; their function is to pick up cholesterol, which is internalized and carried to the liver or steroidogenic organs. Probably due to this role, HDL circulating levels are inversely associated with ASCVD [19], but there is no evidence that increasing these levels could reduce cardiovascular risk [20]. Lipoprotein(a) (Lp(a)) is similar to LDL but contains apolipoprotein Apo(a) in addition to ApoB. This particle, thanks to its small diameter, can pass through the endothelial barrier, provoking atherogenesis. Moreover, because they have a structure similar to plasminogen, pro-coagulant and pro-inflammatory effects have been shown [21]. The association between higher Lp(a) circulating concentrations and increased CVD risk has been assessed [22][23][22,23]; furthermore, a reduction in these levels in patients treated with proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors was shown to reduce CV risk [24][25][24,25]. Atherosclerosis is a long-lasting process, but dyslipidemia has been shown to play a key role at different stages. In the initiation phase, the LDL particles, with a high cholesterol content, accumulate in the innermost layer (intima) of the vessel [26]. Here, these particles undergo an oxidation reaction and are phagocytosed by macrophages, which are transformed into foam cells [26]. As result, a localized inflammatory reaction begins, with the consequent release of cytokines and expression of adhesion molecules, attracting other monocytes circulating in the blood, transforming them into macrophages which in turn become foam cells [26]. Since inflammation plays a pivotal role in the atherosclerotic process, C-reactive protein (CRP) has been chosen as a prognostic marker for cardiovascular risk [27]. Of note, type 1 T helper lymphocytes (producing cytokines such as IFN-γ and TNF) have been found in human atherosclerotic plaques as promotors of atherogenesis, while regulatory T cells seem to mitigate this process [28][29][28,29]. In physiological conditions, the arterial endothelium has intrinsic properties preventing thrombus formation; however, the alteration of endothelium function, which loses its permeability and nitric oxide (NO)-mediated vasorelaxation capacity [30][31][30,31], may contribute to thrombus formation. In addition, other factors, such as flow alterations, may influence atherosclerosis initiation; indeed, plaques tend to form at flow disturbance sites, such as at the branching of vessels [32]. During plaque enlargement, due to high lipid levels, calcification of plaque may occur [33]; if calcification is very extended, plaque disruption and consequent thromboembolic events will be less probable [34]. Finally, plaque evolution and consequent complications depend on fibrous cap thickness and lipid core quantity. A thin fibrous cap determines more vulnerability and probable rupture [35]; when rupture of an atherosclerotic plaque occurs, the consequent exposure of thrombogenic material that is in the core (such as tissue factor) and circulating (thrombin) triggers formation of a thrombus [35]. Otherwise, when plaque has lower inflammatory cells and lipid content, greater collagen matrix content and thicker fibrous cap, a different complication called “plaque erosion” may occur, leading to formation of platelet-rich “white” clots. The complication discussed so far typically occurs in coronary arteries, causing myocardial infarction [36]. In other vessels, atherosclerotic plaque growth may continue uninterrupted until the formation of flow-limiting lesions, causing PAD. In addition to dyslipidemia, other risk factors contribute to the atherosclerotic process. Arterial hypertension causes endothelial dysfunction through shear stress [32], and in addition it increases levels of angiotensin II, which activates the prescription of nuclear factor-κB (NF-κB), responsible for inflammatory pathways [37]. Type 2 diabetes mellitus causes insulin resistance and accumulation of visceral adipose tissue, which contains inflammatory cells and increases circulating pro-inflammatory cytokines levels [5]. In conclusion, several factors are demonstrated favoring plaque formation, but dyslipidemia is certainly the main atherogenesis promoter, on which an intervention is desirable.3. Dyslipidemia and Atherosclerosis in Transplant Recipients
In recent years, advances made in the field of solid organ transplants, as regards surgical techniques, infection prevention, and immunosuppressive therapy, have resulted in increasing survival of transplant recipients. Consequently, recent data have shown an increasing prevalence of CVD, which has become the leading cause of death following kidney [10] and heart transplant [11], and the second leading cause after liver transplant [12][38][39][12,38,39]. It should be considered that kidney transplantation can be a confounding factor, as it is often the final stage of long-lasting CKD, which is itself an important cardiovascular risk factor [40], although this had been underestimated by cardiovascular risk prediction models such as the Framingham Risk Score [41][42][41,42]. The latest European Society of Cardiology (ESC) guidelines on cardiovascular disease prevention have defined patients with moderate CKD as high risk for CVD, and those with severe CKD at very high risk [43]. For other solid organ transplant recipients, among causes of this increase in CVD, it should be considered that dyslipidemia occurs frequently in these patients. The pathophysiological mechanisms through which an increase in circulating LDL levels favors atherosclerosis and consequently ASCVD have been presented in the previous chapter. Prevalence of hyperlipidemia was estimated at 80% in kidney transplant recipients [44], 50% in heart transplant recipients [45], and about 70% in liver transplant recipients [15], compared to about 35% in the general population [46][47][48][49][50][46,47,48,49,50]. Multiple factors contribute to lipid alterations, such as genetic predisposition [44][45][46][44,45,46], dietary habits [51], and age; however, the main effect is due to immunosuppressants, which have shown intrinsic pharmacodynamic properties to cause dyslipidemia and hyperglycemia [52][53][52,53]. In line with these findings, serum total cholesterol concentration is higher in the first 3–6 months after transplantation, when immunosuppressants are administered at higher doses [54]. These drugs are responsible not only for the increase in LDL and triglycerides but are also associated with other atherosclerosis promoter factors. Arterial hypertension is a common side effect of immunosuppressants such as cyclosporine [55] and tacrolimus [56] and has been found in approximately 80% of transplant recipients [57]; corticosteroids, the oldest and most used immunosuppressants, have a well-known ability to cause hyperglycemia and diabetes [58], and this effect has been shown to be enhanced by cyclosporine and tacrolimus [59]. Furthermore, dyslipidemia can cause other complications, in addition to CVD, in solid organ transplant recipients. In kidney transplant recipients there is a higher concentration of oxidized LDL (oxLDL) particles [60], possibly due to increased inflammatory state [61] or higher concentration of pro-inflammatory triglycerides [62]. These higher oxLDL, levels, together with the proven lower HDL concentration, are associated with chronic allograft nephropathy (CAN) [63], which is the main cause of kidney transplant failure [64][65][64,65]. Finally, among the factors promoting atheroma formation and growth, a protracted activation of inflammation in transplant recipients has been demonstrated [66][67][66,67]. A particular consequence of inflammation, the cardiac allograft vasculopathy (CAV), has been observed after heart transplantation [68]. CAV is a particular form of coronary disease, which is responsible for about 10% of deaths after heart transplant [68]; it is distinguished from normal coronary atherosclerosis because these lesions, which appear as concentric intimal hyperplasia obliterating the lumen of the vessel, affect the intramuscular arteries and the microvascular bed [69]. The pathogenesis is mainly due to the formation of antibodies against donor antigens, which trigger an inflammatory response mediated by T lymphocytes directed against donor endothelial cells, which consequently proliferate and occlude the vessel lumen [70][71][70,71]. However, in addition to inflammation, other metabolic factors, including dyslipidemia, are also promoters of CAV [71]. Mechanisms of atherosclerosis in transplant patients are illustrated in Figure 1.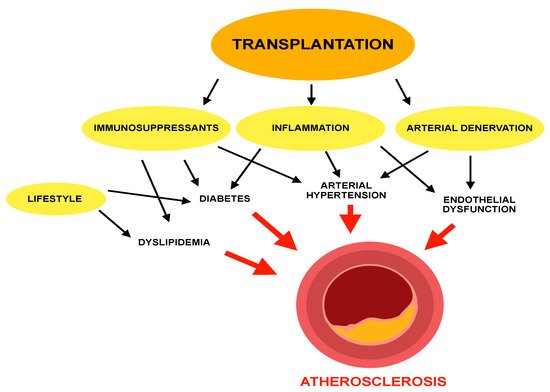
Figure 1.
Mechanisms of atherosclerosis in transplant patients.
