1. A Comparative Look at the Pathogenic RNA Viruses—SARS-CoV-2 and Ebola Virus
The causative pathogen for the COVID-19 global pandemic is SARS-CoV-2, belonging to the family of Coronaviruses which are positive-stranded RNA viruses. Since elucidation of the molecular profile of the virus, the evolution of several variants of concern has occurred
[1]. In contrast, there is comparatively more literature available for Ebola virus disease (EVD) also known as viral hemorrhagic fever. EVD caused by Ebola virus (EBOV) that belongs to the Filoviridae family which comprises six species, of which Zaire Ebola virus, Bundibugyo virus and Sudan virus have caused huge human outbreaks
[2], among which the Zaire Ebola virus is found to be the deadliest.
Most of the emerging RNA viruses capable of infecting humans are found to be of zoonotic nature with known or suspected reservoirs in animals. COVID-19 is believed to originate from the outbreak of SARS-CoV-2 which started in Wuhan, China, probably by a spillover event from an unknown animal host
[3]. In the case of EBOV, wild animals, especially fruit bats are the natural reservoirs
[4], likely spreading to humans upon contact and subsequent entry following breaks in the mucosal surfaces. Further spread occurs as a result of direct contact with the patients and their body fluids or through nosocomial transmission. While in the case of COVID-19, the mode of transmission is primarily through respiratory droplets and aerosols
[5]. Though the rate of transmission is much higher for COVID-19, the mortality rates are greater for EVD.
Both these RNA viruses have an incubation period before the onset of symptoms. Filoviral infection is followed by non-specific flu-like symptoms similar to the prodrome of many other viral diseases. In a retrospective study conducted in non-severe cases of COVID-19, four phases of the disease course have been proposed: a prodromal phase in the first week followed by a manifestation phase in the second week and finally a convalescent phase after three weeks of infection
[6]. Disease progression of EBOV is characterized by maculopapular rashes and the late stage of the disease is indicated by complications of the gastrointestinal, respiratory, vascular and neurological systems, multi-organ failure and subsequent death
[7]. Multi-organ damage followed by death is also a characteristic feature encountered in severe COVID-19
[8].
One of the strategies used by RNA viruses is to condense their genetic information into small genomes, which are translated immediately after viral entry into proteins needed for replication. EBOV has a single linear negative sense RNA of 19kb size, that codes for nucleoprotein (NP), polymerase cofactor VP35, matrix proteins VP40 and VP24, glycoprotein (GP), transcription activator VP30, and an RNA-dependent RNA polymerase (L)
[7][9][10][11][12][7,9,10,11,12]. However, SARS-CoV-2 virus carries one of the largest genomes among RNA viruses, with a size of approximately 30 kb (almost double the size of EBOV genome)
[13]. Two large polyproteins pp1a and pp1ab are formed by translation of the ORF, which in turn lead to production of functional viral RNA polymerase. The four structural proteins of SARS-CoV-2 virus have been well-characterized—Spike (S), Membrane (M), Envelope (E) and Nucleocapsid (N). The spike protein has a receptor binding domain (RBD) which is poorly conserved and is a highly specific target for human antibodies
[14]. Both SARS-CoV-2 and EBOV use a surface glycoprotein for viral entry into the host cell, which then binds to a specific receptor on the host cell, leading to endocytic fusion of the virus. SARS-CoV-2 utilizes the ‘S’ protein
[15] and EBOV makes use of Glycoprotein (GP)
[16][17][18][19][16,17,18,19] for viral entry. The primary host receptor used, in the case of SARS-CoV-2, is ACE-2 which is highly expressed in alveolar and intestinal epithelial cells
[20], while in the case of EVD, the primary host receptor is T-cell immunoglobulin and mucin domain-1 (TIM-1)
[21][22][21,22]. In both cases, the viral entry is followed by replication of the viral genome and propagation of new viral particles that takes place through a series of successive steps.
Structural aspects and mechanisms of pathogenesis associated with SARS-CoV-2 and EBOV have been compared in Table 1.The role of lipids in viral entry and pathogenesis has been a hot topic of research. The involvement of lipids is proposed to occur mainly at three levels—the interaction of viruses with the lipid barrier offered by the host cell membrane, regulation of lipid metabolism to fuel viral replication and stimulation of production of several lipid mediators which can modulate the host immune response. Most enveloped viruses that have a mechanism of endosomal escape make use of apoptotic mimicry when Phosphatidylserine/Phosphatidylserine receptors serve as a route of virus entry. Phosphatidylserine (PS) receptors are demonstrated to facilitate infection of SARS-CoV-2 and hence have been suggested as a therapeutic target
[23][37]. EBOV is also known to make use of the host apoptotic clearance mechanism to augment its entry into target cells by externalizing PS on its surface
[24][25][26][38,39,40]. In both SARS-CoV-2
[23][37] and EBOV
[27][28][41,42], the T cell Ig and mucin domain (TIM) family of proteins is shown to be involved in the above mentioned mechanism. Lipid raft domains rich in cholesterol and sphingolipids, on cellular membranes that play a significant role in viral trafficking and pathogenicity, are also studied in SARS-CoV-2
[29][43] and EBOV Virus
[30][44].
2. Host Anti-Viral Immune Response in SARS-CoV-2 and EBOV Infections
Antiviral immunity in humans is comprised of a rapid nonspecific innate response intended to eliminate the virus, followed by B- and T-cell-mediated virus-specific adaptive responses
[31][45]. In the ensuing sections,
we ha
ve presented a comparison of the host immune response mechanisms
were presented involved in COVID-19 and EVD which would facilitate similar studies on diseases caused by other RNA viruses.
2.1. Defining Innate Immunity
Innate immune responses hold the first line of defense against the attack of any microbial agent. Pathogen associated molecular patterns (PAMPS) derived from the pathogen, recognized by pattern recognition receptors (PRR) alert the innate immune system to the infection. This is followed by release of cytokines which then trigger production of pro-inflammatory molecules resulting in formation of a pro-inflammatory feedback loop
[32][46]. This mechanism has been proposed for SARS-CoV-2
[32][46] and EBOV
[33][47], which in turn leads to a hyperinflammatory immune response known as Cytokine Storm Syndrome (CSS) that needs to be managed by anti-cytokine therapy. Such therapies have been put forward for SARS-CoV-2
[34][48] and EBOV
[35][49]. There are several similarities in the innate immune response generated upon SARS-CoV-2 and EBOV infection, which are illustrated in
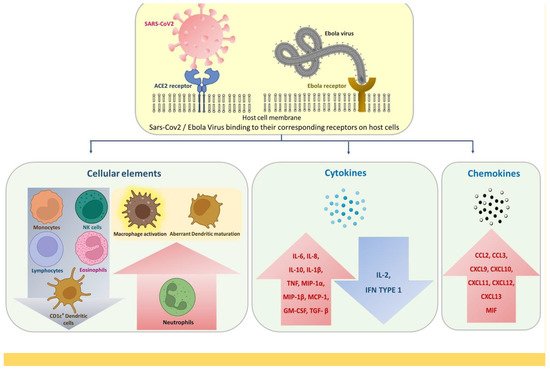
Figure 1.
Figure 1.
Innate immune response alterations common for SARS-CoV-2 and EBOV infections.
Evasion of innate immunity has been proposed to be an immunological mechanism generally used by RNA viruses. Inhibition of type I interferon (IFN) response is identified to be primarily involved in this phenomenon, in SARS-CoV-2
[36][50] as well as EBOV. Mutations in genes involved in the regulation of type I and III IFN immunity leading to production of autoantibodies have been found to be linked with life threatening conditions in COVID-19
[37][51]. Moreover, COVID-19 has been found to become critical in individuals with inborn errors of type I IFN immunity
[37][51]. In case of EBOV, type I IFN signaling is known to be inhibited by the Interferon inhibiting domain (IID) of the viral proteins VP35 and VP24
[38][52]. Interestingly, knockout mice deficient in interferon receptors are being used as experimental murine models for EVD, which underlines the involvement of interferon signaling in susceptibility to Ebola infection
[39][40][53,54].
Neutrophil extracellular traps (NETs), originally described as a potential bacterial killing mechanism, is reported to be involved in the pathogenesis of viral diseases too
[41][55]. NETosis, a regulated cell death mediated by neutrophils can follow the hyperinflammatory response to SARS-CoV-2 infection. Severe infection with SARS-CoV-2 results in release of inflammatory cytokines and chemokines which leads to release of NET, ultimately causing the clearance of the virus
[42][56]. A multi-omics analysis of peripheral blood from EVD survivors also suggests involvement of NET associated proteins in causing tissue damage
[43][57].
Inflammation is one of the major host defense mechanisms against viral infection. The lipid mediator, Prostaglandin E2 is known to play an important role in the associated inflammatory and immune responses
[44][58]. In severe COVID-19, Prostaglandin E2 is shown to mediate impaired immune response
[45][59]. Prostaglandin E is also demonstrated to be upregulated in Filoviral infection in bats
[46][60], pointing towards the significance of prostaglandins in pathogenesis of EVD.
A life-threatening complication associated with both COVID-19 and EVD is disseminated intravascular coagulation (DIC), characterized by activation of coagulation system, leading to generation of microthrombi
[47][61]. The infection of monocytes and macrophages with the virus results in release of tissue factor (TF), which is found to be important in the development of coagulopathy associated with COVID-19
[48][62] and EVD
[49][63].
2.2. Alterations in Adaptive Immune Response
Adaptive immunity has two elements: humoral immunity and cell-mediated immunity, mediated by B-cells and T-cells, respectively. Once the virus enters the cell, activation of naive B cells leads to antibody production, mainly IgM followed by class switching to IgG. At the same time, activation of T-cells leads to further activation of CD4+ (Helper T cells) and CD8+ (Cytotoxic T cells). The cytokines secreted by the CD4+ T-cells facilitate the production of antibodies from B cells. The CD8+ T-cells on the other hand, help in the killing of infected cells. Upon encounter with the cognate antigen, some of B cells enter the germinal center and activate plasma cells leading to specific antibody production, with the help of T cells.
Sette et al.
[50][64] propose that in the case of SARS-CoV-2 infections, the innate immune response is the first line of defense, as has been previously reported for any viral infection, and the adaptive response takes over soon after the waning of the innate response. The contributions of the innate and adaptive immunity become deeper with severity of the infection.
2.2.1. Overview of the Humoral Immune Response
The humoral immune response in viral infection is characterized by the secretion of neutralizing antibodies that protect the host against viruses. The humoral immune response to SARS-CoV-2 has been well studied. In one of the initial studies carried out by Long et al., 100% of patients exhibited anti-viral IgG within 19 days of infection
[51][65]. IgG and IgM antibody titers have been found to be increased during the first 3 weeks after the onset of symptoms
[51][65]. Similar studies have been carried out in EVD, with various patterns of observations. EVD specific IgG and IgM antibodies were found to be very low in patients who died of infection
[52][66]. Analysis of EBOV specific IgG and IgM in 2014–16 Sierra Leone outbreak survivors indicated that humoral response develops in the first week of onset of symptoms and the concentration of antibody increases at the end of the second week of illness. IgG was detected earlier than IgM and the viral load was found to be negatively correlated with antibody titer
[52][66]. IgM response preceding that of IgG response as well as both responses occurring at the same time have been demonstrated in different EVD patients in yet another study
[53][67]. A comparative antibody response curve upon SARS-CoV-2 and EBOV infection has been illustrated in
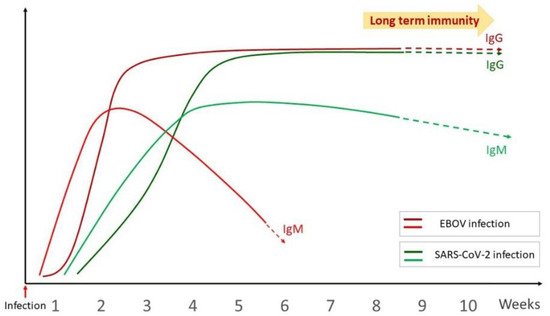
Figure 2.
Figure 2.
Comparative antibody response curve upon SARS-CoV-2 and EBOV infection.
Neutralizing antibodies, which play a significant role in viral clearance, are well correlated with long term anti-viral immunity
[54][68]. Potent neutralizing glycan cap-specific monoclonal antibodies effective against multiple Ebola viruses have been demonstrated in survivors of EVD
[55][69]. Wang et al. has studied the level of neutralizing antibodies in COVID-19 inpatients and convalescent patients and demonstrated its association with age, severity of disease and time after onset of symptoms
[56][70].
Non-neutralizing antibodies are known to play a significant role in humoral immune response. In filoviral infection, non-neutralizing antibodies are also reported to confer protection that is mediated through the Fc domain
[57][71]. This is achieved by antibody-dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells or antibody-dependent cellular phagocytosis (ADCP) of viral particles or infected cells by Fc receptor-bearing cells
[58][72]. A recent report indicates that the protection offered by non-neutralizing antibodies to SARS-CoV-2 are mediated by Fc-mediated effector functions
[59][73] such as phagocytosis.
There are reports that suggest a correlation between antibody kinetics and severity of COVID-19, with delayed antibody response observed in severe disease
[60][74]. Characterization of antibody response to spike protein has revealed the existence of immunological imprinting, which refers to the ability of the immune system to recall existing memory cells developed in response to earlier betacoronavirus infections, rather than stimulating de novo responses
[61][75].
Plasmablasts, known to be involved in formation of memory B-cells and plasma cells play a significant role in humoral immunological memory
[62][76]. An increase in plasmablasts has been reported in COVID-19
[63][64][77,78] and EVD
[53][65][67,79]. RBD specific memory B cell response to SARS-CoV-2 antigen is proved to evolve between 1.3 and 6.2 months after infection, in a study conducted by Gaebler et al.
[66][80]. Assessment of immunological memory in SARS-CoV-2 revealed that the level of IgG against spike protein was stable over more than six months
[67][81]. Presence of EBOV specific memory B cells has also been demonstrated in EVD survivors, which was found to increase with time post-convalescence
[68][82].
Ever since the first recognized Ebola outbreak occurred in 1976, investigators have attempted to assess the long-term antibody kinetics in EVD survivors. Bebell et al. has reported that IgM was found to persist one to six months after infection whereas IgG persisted up to 10 years
[69][83]. Reports also indicate the presence of antibodies specific to Ebola viruses 11 years post-infection
[70][71][84,85]. Another study carried out 40 years after initial viral infection, in survivors of the first Ebola outbreak, interestingly revealed that 50% of the survivors displayed immunoreactivity to viral glycoprotein, nucleoprotein and VP 40, with the ability to neutralize live virus
[72][86]. Similar attempts have been made in COVID-19 where long term kinetics studies reveal that neutralizing antibody responses could exist up to 13 months after SARS-CoV-2 infection
[73][87]. More research is warranted on the antibody kinetics in long term survivors of various outbreaks caused by RNA viruses that could give more insights on long lasting viral immunity.
Adaken et al. carried out an interesting longitudinal follow up study of antibody levels in survivors of 2013–2016 Ebola outbreak which point towards a decay-stimulation-decay model.
HereIn thi
ns study, IgG levels are found to decrease following recovery, which then show a resurgence over a period of time
[74][88]. Another 60 month observational prospective cohort study carried out with a larger sample size comprising 802 EVD survivors also evidenced a similar antibody ebb and flow pattern
[75][89]. This periodic waxing and waning of antibodies observed in EVD, warrants investigation also in the context of COVID-19, since it may indicate sub-clinical de novo antigenic stimulation possibly indicative of viral RNA shed from immunologically privileged sites such as eyes, central nervous system and testes
[76][90].
Difference in immunity with respect to gender has always been a matter of curiosity among the public and researchers. A study carried out in COVID-19 patients revealed a stronger antibody response in females compared to males, and this has been likely responsible for the lower fatality rates observed for females
[77][78][91,92]. In contrast, there is no difference in the risk for SARS-CoV-2 infection between males and females, though severe forms are common among men
[79][93]. It is worth noting that apart from biological reasons, women are more vulnerable to infections such as COVID-19 and EBOV due to occupational and domestic exposures
[80][94].
2.2.2. Landscapes in T-Cell-Mediated Immune Response
T-cells are major contributors to the adaptive immune response, playing diverse roles such as directly killing infected host cells, activation of other immune cells, production of cytokines and regulation of the immune response. T-cell response is very significant in deciding the course and clinical outcome of viral infections. CD4+ and CD8+ T cell subsets participate in T-cell immunity during viral infection; alterations of these two cell subsets have been widely studied in COVID-19 and EVD.
Transient depletion of both CD4
+ and CD8
+ T-cells has been reported in COVID-19 infection
[81][95] and EVD
[82][96]; however, peripheral T-cell counts are preserved in asymptomatic COVID-19 patients
[83][97]. Studies have found lymphopenia to be characteristically associated with critical COVID-19
[84][98], for which several theories have been put forward by researchers. Andre et al. have postulated T-cell apoptosis to be a major reason for T-cell depletion
[85][99]. EVD is also characterized by deficiency in T-cell responses, apoptosis of lymphocytes and lymphopenia
[38][52]. In vitro studies have demonstrated that EBOV binds to CD4
+ T cells which is mediated by the interaction of GP with Toll-like receptor 4 (TLR4). This results in consequent production of Tumor necrosis factor α (TNF α) thereby stimulating apoptosis and necrosis of T lymphocytes
[86][100].
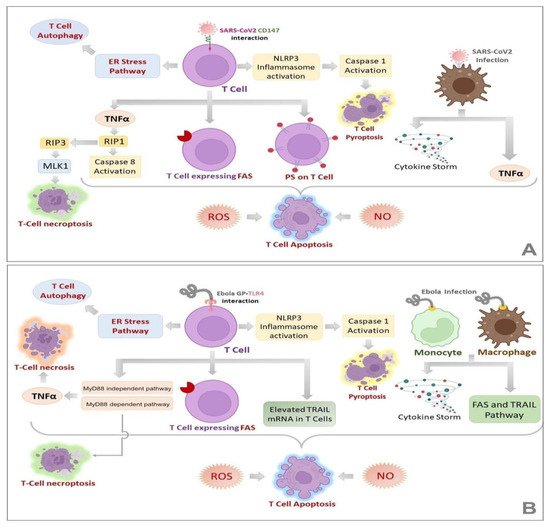
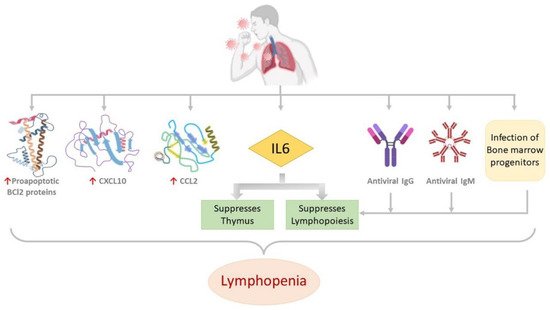
Figure 3A,B illustrate various mechanisms that could lead to lymphopenia in SARS-CoV-2 and EBOV infections, respectively. Quite a few molecular alterations are reported to be associated with lymphopenia in COVID-19 patients, which are illustrated in
Figure 4.
Figure 3. Mechanisms of Lymphopenia in SARS-CoV-2 (A) and EBOV (B) infections. Lymphopenia, a common feature of COVID-19 and EVD, is characterized by different routes of T-cell death such as apoptosis, necrosis, necroptosis and autophagy. (A,B) demonstrate the known mechanisms involved in each of these pathways in COVID-19 and EVD, respectively. It is noteworthy that many of these alterations are common for both the diseases, the fact that can be exploited for the design of novel pan-viral therapies.
Figure 4. Molecular alterations associated with lymphopenia in COVID-19 patients. Proapoptotic BCl2 proteins, CXCL10, CCL2 and IL6 are upregulated. Antiviral antibodies IgG and IgM, infection of bone marrow progenitors and the upregulated IL6 suppresses lymphopoiesis. IL6 also suppresses the thymus. All these are reported to be associated with lymphopenia in COVID-19 patients.
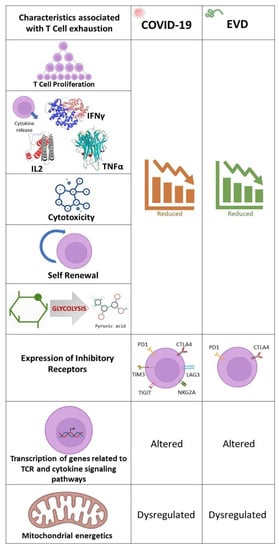
T-cell exhaustion, which is a dysfunction or physical elimination of antigen specific T-cells
[87][101] is a characteristic of several viral infections and has been reported in COVID-19
[88][89][102,103] and EVD
[90][104]. The overexpression of Programmed cell death-1 (PD-1) and Cytotoxic T-lymphocyte-Associated protein 4 (CTLA-4), co-inhibitory receptors
[91][105] is a common feature of T-cell exhaustion found in EBOV
[92][106] and SARS-CoV-2
[93][107] infections. The percentage of CD4
+ and CD8
+ T cells expressing PD-1 and CTLA-4 has been found to be elevated in fatal EVD cases and reduced in survivors
[92][106]. Additional inhibitory receptors such as Natural Killer group 2 member A (NKG2A)
[94][108], TIM-3
[95][109], T cell immunoglobulin and ITIM domain (TIGIT)
[96][110] and Lymphocyte-activation gene 3 (LAG-3)
[97][111] have been hypothesized to be responsible for T-cell exhaustion in COVID-19. The various factors associated with T-cell exhaustion encountered in COVID-19 and EVD are illustrated in
Figure 5.
Figure 5. Factors associated with T-cell exhaustion in COVID-19 and EVD. Proliferation of T-cells, release of cytokines, cytotoxic and self-renewal capabilities of T-cells and glycolysis are commonly found to be reduced in COVID-19 and EVD. Additionally, T-cells express exhaustion markers, of which PD-1 and CTLA4 are common. Alteration of transcription of genes related to TCR and cytokine signaling pathways and dysregulation of mitochondrial energetics are also a common feature of T-cell exhaustion in COVID-19 and EVD.
2.2.3. Long Term Anti-Viral Immunity
Two major concerns related to the long-term management of viral infections are recurrence of symptoms and reinfection. The persistence of virus at immunologically privileged body sites has been reported to cause recurrence of symptoms, after recovery, in COVID-19 and EVD patients. Investigators have detected EBOV in the urine, semen
[98][112], conjunctiva, sweat, vaginal and rectal fluids
[99][113], aqueous humor
[100][114], breast milk and CSF
[101][115] of recovered patients
[102][116]. In addition, EVD survivors have reported contracting conditions such as uveitis
[100][114] and neurological symptoms post-recovery
[101][115]. The presence of SARS-CoV-2 virus in various body fluids has also been extensively studied and reviewed by some investigators
[103][104][117,118].
In view of these facts, the survivors of EVD and COVID-19 may also pose a threat of transmission of the virus and cause new outbreaks. Such a resurgence of EBOV has been proven by the genome analysis of patients seven years after the viral outbreak in Guinea
[105][119]. Evidences also indicate sexual transmission of EBOV from survivor to partner, several days after recovery
[106][107][108][120,121,122]. The possibility of re-emergence of COVID-19 needs to be explored in this direction.
Reinfection by viruses depends on the immunity of recovered patients which may vary from person to person, resulting in variability of individual host susceptibility. Molecular phylogeny analysis of human infecting coronaviruses points to a probability of reinfection with SARS-CoV-2 between 3 months and 5.1 years after peak antibody response
[109][123]. An evaluation of reinfection rates of COVID-19 reveals that only as little as 1% of the people who were previously infected, reported with reinfection
[110][124]. However, the reinfection rate has rocketed to 10% since the emergence of Omicron, which supports the popular belief that Omicron is able to evade the immune responses induced by vaccination or previous infection
[111][125]. In the case of EVD, confirmed reinfection cases in humans have not yet been reported though it is theoretically feasible
[112][126]. However, reinfection has been demonstrated in mice and non-human primates with partial immunity
[113][114][127,128].
Long-lived self-renewing T-cell memory is a fundamental property that results in swift and vigorous adaptive immunity on re-exposure to the same pathogen
[115][129]. Both CD4
+ and CD8
+ are found to contribute to EBOV Glycoprotein (GP) specific T cell memory
[116][130]. SARS-CoV-2 specific memory T cells have been found to be preserved in infected individuals irrespective of the severity of the disease
[117][131]. Heterologous immunity, where memory T-cells generated upon encounter with a pathogen providing immunity against other novel pathogens has also been recently reported in SARS-CoV-2 patients, which could lead to varying severity outcomes
[118][132]. This has been evidenced by the identification of pre-existing non-spike memory T-cells in SARS-CoV-2-naive contacts that offered them protection against COVID-19
[119][133]. A very striking finding is the production of virus specific T memory stem cells which are unique subcategories of memory T cells with self-renewing and multipotent abilities
[120][134]. The presence of stem cells such as memory T cells has also been documented in both EVD
[116][121][130,135] and SARS-CoV2
[117][131] convalescents.
The repercussions of emerging viral infections in survivors are a matter of great concern. EVD survivors are found to suffer from various illnesses including impotence, musculoskeletal pain, bleeding, ocular diseases, hearing loss, psychological problems which encompasses Post Ebola virus disease syndrome (PEVD)
[122][123][136,137]. Similarly, post-acute COVID-19 syndrome characterized by persistent symptoms and long term complications such as dyspnea, fatigue, muscular weakness, joint pain, thromboembolism and anxiety are reported to occur from 4 weeks post-onset of symptoms
[124][138]. Autoantibodies developed against self-antigens post-viral infection are documented to be associated with the above mentioned post-viral syndromes
[125][126][127][139,140,141].
2.3. Risk Factors That May Influence the Immune Mechanism
Several host factors such as genetic susceptibility, age and sex, co-morbidity and immune compromised states are found to influence the immune responsiveness and hence the outcome of the viral infection. Right from the initial days of the pandemic, presence of co-morbid conditions such as chronic pulmonary obstruction, obesity, and hypertension have been linked to the severity of COVID-19
[128][142]. The compromised immune response is thought to be responsible for the very high increase in mortality and severity of COVID-19 seen among diabetics
[129][143]. Moreover, disease-modifying therapies (DMTs) used for the management of comorbid conditions in COVID-19 have been shown to diminish the immune response
[130][131][144,145]. Age has also been well studied as an immunological determinant of COVID-19 disease severity
[132][146].
In the case of EVD, age and sex are not found to have any association with the risk of getting infected
[133][147]. However, studies on the Sierra Leone outbreak indicate that the incidence rate increased with age and the median age of confirmed EVD cases was 28. Additionally, half of the infected cases were females
[134][148]. As for coinfection, there is a dispute over association of
Plasmodium species parasitemia with the prospects of surviving EBOV infection
[135][149]. Such findings have been discussed in a systematic review by Edwards et al.
[136][150]. Studies carried out in murine models warrant further research to prove the effect of
Plasmodium coinfection on survival from EVD
[137][151].