You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Ines Martic.
The human skin is exposed daily to different environmental factors such as air pollutants and ultraviolet (UV) light. Air pollution is considered a harmful environmental risk to human skin and is known to promote aging and inflammation of this tissue, leading to the onset of skin disorders and to the appearance of wrinkles and pigmentation issues. Besides this, components of air pollution can interact synergistically with ultraviolet light and increase the impact of damage to the skin.
- Air Pollution
- Particulate Matter
- Cigarette Smoke
- Skin Aging
1. Introduction
Pollution is defined as an environmental contamination by chemical, biological, or physical substances which can affect human health and ecosystems. Air pollutants are composed of organic and inorganic substances which are introduced into the atmosphere by residential wood heating, tobacco smoking, transportation, and industry, among other sources [78][1]. The composition of atmospheric pollution can also vary depending on the time of day, seasons, human activity, and geographic location [8,9][2][3]. Several reports suggest that air pollutant components are direct contributors to the process of aging [5,11,79,80,81,82][4][5][6][7][8][9]. Human exposition to air pollution contributes to increased mortality and hospital stays. The effects of air pollution can range from nausea, difficulty in breathing, skin irritation, birth defects, and reduced activity of immune system, to cancer. Results obtained from research with animal models and epidemiological studies suggest that the cardiovascular and respiratory system are the main affected systems by air pollution [10]. In the lungs, infiltration of air pollutant components, especially small particles which can reach bronchial tubes and deep lung, can induce a systemic immune response due to increased expression of IL-1, IL-6, IL-8, and monocyte chemoattractant protein-1 in macrophages and lung epithelial cells, leading to the development of respiratory diseases [5][4].
The skin can be affected by air pollution in two ways. Either directly, through the uptake of the air pollutants by the skin, especially by intrusions formed by hair follicles in the stratum corneum [5][4], or indirectly by the uptake of particles by the lungs which are further transported by the blood to the skin [9][3]. Exposure of the skin to urban air pollution activates mechanisms of cell detoxification, which, if active over a longer period of time, can lead to DNA and protein damage, elevated ROS levels and lipid peroxidation, resulting in skin alterations, such as impaired barrier function, pigment spots, wrinkles, and decreased skin hydration [83,84][11][12]. Additionally, air pollution induces inflammation, activates the AhR pathway, and leads to alterations of the skin microbiome [85,86][13][14]. Given the strong link between low-grade systemic inflammation and biological aging, it is possible that exposition of the skin to air pollution leads to premature cellular senescence and, consequently, to skin aging [85][13]. The literature regarding the effects of air pollution on different ethnic skin types is still elusive. The few broad ethnic studies show that exposition of the skin to air pollution mainly induces increased oxidative stress leading to skin pigmentation disorders and wrinkle formation [2,11,72][5][15][16]. Information on the effects of air pollution on different skin types is still lacking and therefore the awareness of different ethnicities in this field of research needs more attention.
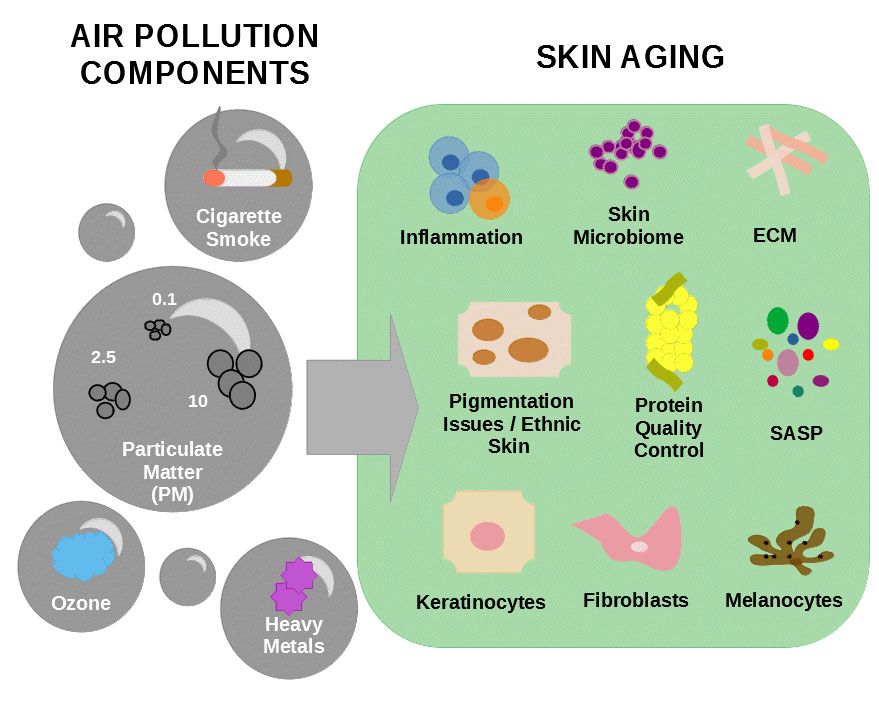
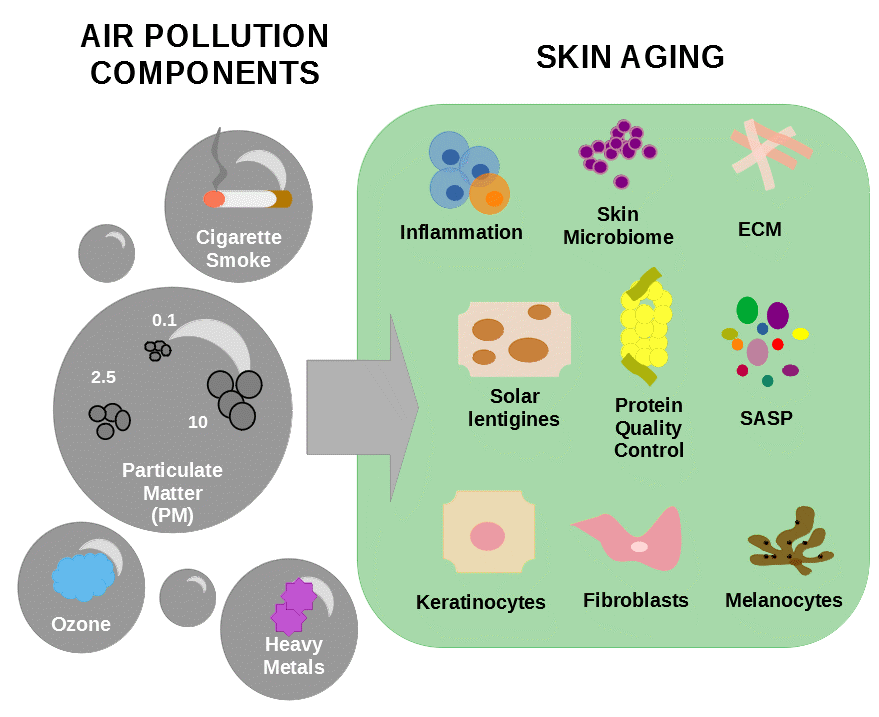
2. Particulate Matter
Particulate matter (PM) is one of the main components of air pollution and is defined as a mixture of gas containing liquid and/or solid droplets varying in size and composition [86,88][14][17]. Particles contained in PM include substances such as metals, minerals, organic toxins, tobacco smoke, pollen, allergens, and smog [89][18]. These particles are classified according to their aerodynamic diameter (Dp) in three categories: particles with Dp bellow 10 µm are named “coarse particles” or PM 10 and include components of dust, soil, and dusty emission from industries; particles with Dp between 0.1 and 2.5 µm are called “particle matter” or PM 2.5 and are mainly derived from open fires, automobile exhausts, and power plants [90][19]; and particles with Dp bellow 0.1 µm are called “ultrafine particles” or PM 0.1 and mainly consist of emissions of diesel-powered engines [86][14]. The aerodynamic diameter of the particles is an important determinator of their ability to enter the body by alveolar–capillary barrier and travel across the blood [91][20]. The particle matter and ultrafine particles can penetrate the body either by systemic distribution through the blood circulation after entering the lungs’ alveoli or by infiltrating the skin through hair follicles [92][21]. Recently, in contrast with what was believed before, it was demonstrated that coarse particles can also penetrate the stratum corneum or the respiratory system [53,93][22][23].
Exposure to particulate matter can cause cardiovascular and respiratory diseases, allergies, and cancer through different mechanisms [83,94][11][24]. For instance, PM 2.5 exposure induces endoplasmatic reticulum stress response and apoptosis in the lung and liver of mice and this mechanism is believed to be one possible explanation for the development of metabolic, respiratory, and cardiovascular diseases in humans exposed to air pollution [95][25]. A study in mice showed that PM inhalation activates the TNF-α driven systemic inflammation and results in an impaired cardiac function which to a certain extent was prevented by a TNF-α inhibitor called inflimiximab [96][26]. In skin diseases such as psoriasis, the blockage of TNF-α leads to detrimental side-effects such as increased risk of infections and malignancies as well as to the formation of new and more psoriatic skin lesions [97][27].
A cross-sectional study which investigated the correlation of particulate matter exposure and aging demonstrated that leukocytes of peripheral blood from elderly humans exposed to daily high concentrations of PM 2.5 presented decreased telomere length, lower mitochondrial DNA content, and reduced sirtuin-1 expression [98][28]. In another study it was shown that exposure of human nasal epithelial cells to PM 2.5 leads to ROS production, degradation of tight junction proteins such as occludin, claudin-1, and E-cadherin, leading to sinonasal diseases through disrupted tissue integrity and permeability [45,99][29][30]. Similar downregulation of tight junctions as well as keratins and filaggrin were observed in pork skin in response to PM 2.5 exposure, leading to increased skin permeability [100,101][31][32]. Altogether, these studies demonstrate that short- and long-term exposure to PM can induce features of premature aging.
Particularly in the skin, PM 2.5 induces different detrimental processes such as DNA damage and lipid peroxidation. Exposure of the skin to PM results in the formation of senile lentigines, increased formation of ROS, and promotes the release of pro- inflammatory cytokines which all lead to accelerated skin aging and increased susceptibility to pathogen invasion [5][4]. ROS generation, induced by exposure of the skin to air pollution, can induce MMPs expression and increase their activity, especially MMP-1 and MMP-3 which are known to accelerate the skin aging process by degrading collagen and elastin. Additionally, the decreased expression of transforming growth factor (TGF) β, and reduced synthesis of collagen type 1 α chain (COL1A1, COL1A2) and elastin by fibroblasts are other contributors to the formation of wrinkles and skin aging induced by PM [80,82,102][7][9][33].
In human keratinocytes and mouse skin tissue, PM induced the expression of demethylases such as DNA demethylase 1 (TET1) and decreased the expression of DNA methylation-related proteins such as (DNMT)-1 and -3. These changes in the methylation pattern of DNA induced “skin senescence phenomenon” are characterized by features such as hyperkeratotic epidermis accompanied by the increased expression of keratin-10, an epidermal differentiation marker, and proliferating cell nuclear antigen, a proliferation marker [79][6]. These results demonstrate that PM-induced skin aging can be triggered by epigenetic modifications which could give rise to new strategies for therapeutics against skin aging.
Skin cells exposed to diesel particulate extract (DPE), which mainly contains PM and PAH [103][34], displayed dysregulation of proteins and lipids important for the maintenance of skin integrity, for the regulation of skin hydration and oxidative stress, such as NADPH oxidase (NOX), ceramide, plakins, transglutaminases, cystatins, and filaggrin [57,80][7][35]. Furthermore, DPE impaired mitochondrial oxidative phosphorylation and cell migration in these cells. These effects could be partially avoided or restored by the treatment of the cells with the antioxidant vitamin E [57][35].
PM causes inflammation in the skin through increased IL-8, MMP-1, ROS production, and neutrophil infiltration in the deep dermis [104][36]. Fibroblasts cultivated with conditioned medium obtained from immortalized human keratinocytes (HaCaT) treated with PM displayed increased nuclear translocation of p65 and p50 as well as increased ROS production, morphological changes, and secretion of SASP components including prostaglandin E2, COX-2, TNF-α, IL-1β, and IL-6 [93][23]. It is well established that the expression and release of TNF-α is increased upon UV exposure [105][37] as well in some skin diseases such as vitiligo [106][38].
Other studies have shown that the exposition of HaCaT cells or reconstructed human epidermis to PM induced upregulation of NF-κB, COX-1 as well as IL-1α leading to skin barrier dysfunction [81][8]. In addition, an increased autophagic activity shown by the turnover of the light chain 3 Ι (LC3) to LC3 II, expression of p62, and PM internalization in the autolysosomes was observed after exposure of fibroblasts to air pollutants such as PM [107,108][39][40].
The PM particles can transport organic chemicals and metals into cells which when localizing in the mitochondria can directly generate ROS [2][15]. Mostly, PM particles contain PAH which can penetrate the skin. PAH is an activator of AhR in melanocytes and keratinocytes and exposition of the skin to this chemical can influence epidermal turnover, melanogenesis, and differentiation [9,86][3][14]. PAH have synergistic effect with UVA and both contribute to skin carcinogenesis and to the appearance of skin pigmentation disorders such as senile lentigines [5,53,70,92,109,110][4][21][22][41][42][43]. Interestingly, exposure of melanocytes to PM induces apoptosis through cytochrome C release and activation of caspase-3 [111][44]. These events are linked to the disappearance of melanocytes which is considered one of the main pathogenic mechanisms of vitiligo.
The effects of PM 2.5 and 10 are linked to different skin diseases such as AD and allergies [45,112][29][45]. Several studies have demonstrated that AD skin, as well as healthy skin exposed to PM, display skin barrier disruption due to decreased expression of epidermal structural proteins such as filaggrin, E-cadherin, and cytokeratins [43,44,113][46][47][48]. Additionally, it is suggested that AD can be influenced by the exposure to air pollution resulting in imbalance of immune cell response and IgE production, activation of AhR/NF-κB, and the generation of ROS, and these effects can be prevented by improved air quality [5,44,85][4][13][47].
3. Cigarette Smoke
Cigarette smoke is composed of different chemical substances including reactive oxygen species, carbon monoxide, and reactive nitrogen species. Smoking is associated with lung cancer as well as with cutaneous squamous cell carcinoma. PAH, one important component of cigarette smoke, is a major contributor to cancer. PAH induces AhR and subsequently CYP1A1 which leads to the formation of DNA adducts and can result in cancer development [114,115][49][50].
The effects of cigarette smoke are cumulative and associated with the appearance of signs of premature aging, especially in the facial skin. Among them the most common are deep wrinkles, dryness, leathery texture, sagging, premature graying, and orange to purple discoloration of the skin [8,53,88][2][17][22]. Exposition of the skin to cigarette smoke induces oxidative stress and the impairment of the antioxidant system. The consequences are transepidermal water loss, lipid peroxidation, cell death, and degeneration of connective tissue by MMP-1 and -3 [80,114,116][7][49][51]. In keratinocytes, cellular redox homeostasis and colony-forming potential was affected in response to exposure to tobacco smoke components such as PAH and PM [92][21]. In humans, a study comparing smokers and nonsmokers revealed the occurrence of shorter telomeres, a sign of cellular senescence, in peripheral white blood cells of smokers [98][28]. Synergistic effects may occur upon the combined exposure to UV and cigarette smoke leading to epidermal barrier disruption, increased erythema, and decreased elasticity [8,53,88][2][17][22]. Furthermore, a three months’ whole body exposure of mice to tobacco smoke caused premature aging evidenced by the loss of collagen as well as hair loss and premature graying due to increased apoptosis and decreased melanogenesis, respectively [117][52]. Additionally, the combined exposure of tobacco smoke with ultraviolet light, exacerbated the effects of aging by the upregulation of p16 expression. These effects could be prevented by N-acetylcysteine (NAC) suggesting that premature aging triggered by cigarette smoke exposition is driven by ROS [5][4]. Other studies showed that exposure of skin cells, ex vivo skin biopsies, or reconstructed skin to cigarette smoke leads to production of pro-inflammatory cytokines and MMPs, lipid peroxidation, and downregulation of differentiation proteins such as loricrin and this, in turn, resulting in the impairment of skin barrier structure and function [114,115,118][49][50][53].
Besides the consequences of topical exposition of the skin to pollution, the inhalation of pollution has systemically effects to the skin. In a recent in vivo study, it was demonstrated that chronic inhalation of tobacco smoke leads to changes in the composition and deposition of elastin and fibrillin-rich microfibrils in the dermis which is accompanied by a higher stiffness of the skin [119][54].
In a study using a model of skin aging induced by tert-butyl hydroperoxide (tBHP), Wedel and colleagues showed that skin exposed to this chemical displayed downregulation of collagen synthesis and the increased secretion of MMPs. tBHP is categorized as an oxidative stress inducer which leads to the accumulation of intracellular ROS and deplete the antioxidant mechanisms and can be used as a proxy to study mechanisms that recapitulate the exposition of the skin to environmental stressors such as cigarette smoke [61][55].
4. Ozone
Stratospheric ozone has a protective role for earth-living organisms by filtering UV radiation. Ozone (O3) in the troposphere reaches the skin surface and reacts with molecules such as lipids and proteins in the stratum corneum [9,120][3][56]. In general, ozone causes oxidative stress, increased lipid peroxidation, AhR induction, and depletion of Vitamins C and E in human skin [121,122][57][58]. A report has shown that in human keratinocytes, exposure to ozone leads to lactate dehydrogenase release, reduced cell proliferation, lipid peroxidation, and NF-κB activation and these effects can be prevented by pretreatment with antioxidant mixtures [123][59]. Another study has shown that the combination of UVA and ozone has a synergistic effect and causes increased oxidative stress of the skin and Nrf2 expression in keratinocytes leading to skin inflammation, formation of wrinkles, and pigment spots [8,9,104][2][3][36]. In human skin O3 leads to activation of NF-κB, MMP-9, COX-2, and lipid peroxidation in the epidermis, whereas in the dermis expression of collagen-1 and -3 are downregulated. These consequences are accelerating the appearance of skin aging signs including the formation of wrinkles and senile lentigines as well as affecting wound healing [124,125][60][61].
45.4. Heavy Metals
The earth crest is formed by different components, among them heavy metals such as chromium, lead, cadmium, silver, nickel, mercury, manganese, and vanadium [8][2]. These metals can contaminate the water and food supply and cannot be degraded or destroyed. These substances are important trace elements for the maintenance of metabolic reactions but in higher concentrations they become toxic to the human body [10]. Heavy metals from industrial fumes reach plants through acidic rain. For instance, tobacco leaves are rich in cadmium which, when inhaled, cannot be excreted from the human body and leads to long term effects and damage to the lungs, kidneys, and bones [78][1]. A study showed that keratinocytes and skin explants exposed to dust particles containing heavy metals or heavy metals alone displayed increased expression of the pro-inflammatory cytokines IL-6, IL-8, caspase-14, and granulocyte macrophage colony-stimulating factor which are known to alter epidermal differentiation, ECM, apoptosis, DNA damage, lipid peroxidation, and skin immunity resulting in cutaneous inflammation and inflammation skin disorders [86,126][14][62].
References
- Numan, M.; Brown, J.; Michou, L. Impact of Air Pollutants on Oxidative Stress in Common Autophagy-Mediated Aging Diseases. Int. J. Environ. Res. Public Health 2015, 12, 2289–2305.
- Puri, P.; Nandar, S.; Kathuria, S.; Ramesh, V. Effects of Air Pollution on the Skin: A Review. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 415.
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and Skin: From Epidemiological and Mechanistic Studies to Clinical Implications. J. Dermatol. Sci. 2014, 76, 163–168.
- Kim, K.E.; Cho, D.; Park, H.J. Air Pollution and Skin Diseases: Adverse Effects of Airborne Particulate Matter on Various Skin Diseases. Life Sci. 2016, 152, 126–134.
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Marini, A.; Jaenicke, T.; Aue, N.; Welss, T.; Uthe, I.; Krutmann, J. Air Pollution-induced Tanning of Human Skin*. Br. J. Dermatol. 2021, 185, 1026–1034.
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.-M.; Park, C.O.; Hyun, J.W. Particulate Matter-Induced Senescence of Skin Keratinocytes Involves Oxidative Stress-Dependent Epigenetic Modifications. Exp. Mol. Med. 2019, 51, 1–14.
- Shin, K.-O.; Uchida, Y.; Park, K. Diesel Particulate Extract Accelerates Premature Skin Aging in Human Fibroblasts via Ceramide-1-Phosphate-Mediated Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2691.
- Lee, C.-W.; Lin, Z.-C.; Hu, S.C.-S.; Chiang, Y.-C.; Hsu, L.-F.; Lin, Y.-C.; Lee, I.-T.; Tsai, M.-H.; Fang, J.-Y. Urban Particulate Matter Down-Regulates Filaggrin via COX2 Expression/PGE2 Production Leading to Skin Barrier Dysfunction. Sci. Rep. 2016, 6, 27995.
- Park, S.-Y.; Byun, E.; Lee, J.; Kim, S.; Kim, H. Air Pollution, Autophagy, and Skin Aging: Impact of Particulate Matter (PM10) on Human Dermal Fibroblasts. Int. J. Mol. Sci. 2018, 19, 2727.
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367.
- Patatian, A.; Delestre-Delacour, C.; Percoco, G.; Ramdani, Y.; Di Giovanni, M.; Peno-Mazzarino, L.; Bader, T.; Bénard, M.; Driouich, A.; Lati, E.; et al. Skin Biological Responses to Urban Pollution in an Ex Vivo Model. Toxicol. Lett. 2021, 348, 85–96.
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne Particle Exposure and Extrinsic Skin Aging. J. Investig. Dermatol. 2010, 130, 2719–2726.
- Damevska, K.; Simeonovski, V.; Darlenski, R.; Damevska, S. How to Prevent Skin Damage from Air Pollution Part 2: Current Treatment Options. Dermatol. Ther. 2021, 34, e15132.
- Mancebo, S.E.; Wang, S.Q. Recognizing the Impact of Ambient Air Pollution on Skin Health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332.
- Vierkötter, A.; Krutmann, J. Environmental Influences on Skin Aging and Ethnic-Specific Manifestations. Dermato-Endocrinology 2012, 4, 227–231.
- Vierkötter, A.; Hüls, A.; Yamamoto, A.; Stolz, S.; Krämer, U.; Matsui, M.S.; Morita, A.; Wang, S.; Li, Z.; Jin, L.; et al. Extrinsic Skin Ageing in German, Chinese and Japanese Women Manifests Differently in All Three Groups Depending on Ethnic Background, Age and Anatomical Site. J. Dermatol. Sci. 2016, 83, 219–225.
- Drakaki, E.; Dessinioti, C.; Antoniou, C.V. Air Pollution and the Skin. Front. Environ. Sci. 2014, 2, 11.
- Bae, Y.J.; Park, K.Y.; Han, H.S.; Kim, Y.S.; Hong, J.Y.; Han, T.Y.; Seo, S.J. Effects of Particulate Matter in a Mouse Model of Oxazolone-Induced Atopic Dermatitis. Ann. Dermatol. 2020, 32, 496.
- Pan, S.; Qiu, Y.; Li, M.; Yang, Z.; Liang, D. Recent Developments in the Determination of PM2.5 Chemical Composition. Bull. Environ. Contam. Toxicol. 2022, 108, 819–823.
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory Effects of Particulate Matter Air Pollution. Environ. Sci. Pollut. Res. 2020, 27, 42390–42404.
- Soeur, J.; Belaïdi, J.-P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-Pollution Stress in Skin: Traces of Pollutants (PAH and Particulate Matter) Impair Redox Homeostasis in Keratinocytes Exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169.
- Burke, K.E. Mechanisms of Aging and Development—A New Understanding of Environmental Damage to the Skin and Prevention with Topical Antioxidants. Mech. Ageing Dev. 2018, 172, 123–130.
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.-S.; Vaas, A.P.J.P.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, W.; Ahn, G.; Lee, D.-S.; et al. A Keratinocyte and Integrated Fibroblast Culture Model for Studying Particulate Matter-Induced Skin Lesions and Therapeutic Intervention of Fucosterol. Life Sci. 2019, 233, 116714.
- Estrella, B.; Naumova, E.N.; Cepeda, M.; Voortman, T.; Katsikis, P.D.; Drexhage, H.A. Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies. Int. J. Environ. Res. Public Health 2019, 16, 2347.
- Laing, S.; Wang, G.; Briazova, T.; Zhang, C.; Wang, A.; Zheng, Z.; Gow, A.; Chen, A.F.; Rajagopalan, S.; Chen, L.C.; et al. Airborne Particulate Matter Selectively Activates Endoplasmic Reticulum Stress Response in the Lung and Liver Tissues. Am. J. Physiol. Physiol. 2010, 299, C736–C749.
- Marchini, T.; D’Annunzio, V.; Paz, M.L.; Cáceres, L.; Garcés, M.; Perez, V.; Tasat, D.; Vanasco, V.; Magnani, N.; Gonzalez Maglio, D.; et al. Selective TNF-α Targeting with Infliximab Attenuates Impaired Oxygen Metabolism and Contractile Function Induced by an Acute Exposure to Air Particulate Matter. Am. J. Physiol. Circ. Physiol. 2015, 309, H1621–H1628.
- Mylonas, A.; Conrad, C. Psoriasis: Classical vs. Paradoxical. The Yin-Yang of TNF and Type I Interferon. Front. Immunol. 2018, 9, 2746.
- Pieters, N.; Janssen, B.G.; Dewitte, H.; Cox, B.; Cuypers, A.; Lefebvre, W.; Smeets, K.; Vanpoucke, C.; Plusquin, M.; Nawrot, T.S. Biomolecular Markers within the Core Axis of Aging and Particulate Air Pollution Exposure in the Elderly: A Cross-Sectional Study. Environ. Health Perspect. 2016, 124, 943–950.
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial Barrier Hypothesis: Effect of the External Exposome on the Microbiome and Epithelial Barriers in Allergic Disease. Allergy 2022, 77, 1418–1449.
- Zhao, R.; Guo, Z.; Zhang, R.; Deng, C.; Xu, J.; Dong, W.; Hong, Z.; Yu, H.; Situ, H.; Liu, C.; et al. Nasal Epithelial Barrier Disruption by Particulate Matter ≤2.5 Μm via Tight Junction Protein Degradation. J. Appl. Toxicol. 2018, 38, 678–687.
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of Airborne Particulate Matter on Skin: A Systematic Review from Epidemiology to in Vitro Studies. Part. Fibre Toxicol. 2020, 17, 35.
- Pan, T.-L.; Wang, P.-W.; Aljuffali, I.A.; Huang, C.-T.; Lee, C.-W.; Fang, J.-Y. The Impact of Urban Particulate Pollution on Skin Barrier Function and the Subsequent Drug Absorption. J. Dermatol. Sci. 2015, 78, 51–60.
- Kim, S.; Kim, J.; Lee, Y.I.; Jang, S.; Song, S.Y.; Lee, W.J.; Lee, J.H. Particulate Matter-induced Atmospheric Skin Aging Is Aggravated by UVA and Inhibited by a Topical L-ascorbic Acid Compound. Photodermatol. Photoimmunol. Photomed. 2022, 38, 123–131.
- Long, E.; Schwartz, C.; Carlsten, C. Controlled Human Exposure to Diesel Exhaust: A Method for Understanding Health Effects of Traffic-Related Air Pollution. Part. Fibre Toxicol. 2022, 19, 15.
- Rajagopalan, P.; Jain, A.P.; Nanjappa, V.; Patel, K.; Mangalaparthi, K.K.; Babu, N.; Cavusoglu, N.; Roy, N.; Soeur, J.; Breton, L.; et al. Proteome-Wide Changes in Primary Skin Keratinocytes Exposed to Diesel Particulate Extract—A Role for Antioxidants in Skin Health. J. Dermatol. Sci. 2018, 91, 239–249.
- Molina-García, M.; Malvehy, J.; Granger, C.; Garre, A.; Trullàs, C.; Puig, S. Exposome and Skin. Part 2. The Influential Role of the Exposome, Beyond UVR, in Actinic Keratosis, Bowen’s Disease and Squamous Cell Carcinoma: A Proposal. Dermatol. Ther. 2022, 12, 361–380.
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. TNF-α Production in the Skin. Arch. Dermatol. Res. 2009, 301, 87–91.
- Singh, M.; Mansuri, M.S.; Kadam, A.; Palit, S.P.; Dwivedi, M.; Laddha, N.C.; Begum, R. Tumor Necrosis Factor-Alpha Affects Melanocyte Survival and Melanin Synthesis via Multiple Pathways in Vitiligo. Cytokine 2021, 140, 155432.
- Kim, H.; Park, S.-Y.; Moon, S.; Lee, J.; Kim, S. Autophagy in Human Skin Fibroblasts: Impact of Age. Int. J. Mol. Sci. 2018, 19, 2254.
- Yoon, S.; Lim, C.; Chung, H.-J.; Kim, J.-H.; Huh, Y.; Park, K.; Jeong, S. Autophagy Activation by Crepidiastrum Denticulatum Extract Attenuates Environmental Pollutant-Induced Damage in Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 517.
- Serre, C.; Busuttil, V.; Botto, J.-M. Intrinsic and Extrinsic Regulation of Human Skin Melanogenesis and Pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347.
- Mokrzyński, K.; Krzysztyńska-Kuleta, O.; Zawrotniak, M.; Sarna, M.; Sarna, T. Fine Particulate Matter-Induced Oxidative Stress Mediated by UVA-Visible Light Leads to Keratinocyte Damage. Int. J. Mol. Sci. 2021, 22, 10645.
- Dimitrov, A.; Zanini, M.; Zucchi, H.; Boudah, S.; Lima, J.; Soeur, J.; Marrot, L. Vitamin C Prevents Epidermal Damage Induced by PM-associated Pollutants and UVA1 Combined Exposure. Exp. Dermatol. 2021, 30, 1693–1698.
- Suo, D.; Zeng, S.; Zhang, J.; Meng, L.; Weng, L. PM2.5 Induces Apoptosis, Oxidative Stress Injury and Melanin Metabolic Disorder in Human Melanocytes. Exp. Ther. Med. 2020, 19, 3227.
- Hieda, D.S.; Anastacio da Costa Carvalho, L.; Vaz de Mello, B.; de Oliveira, E.A.; Romano de Assis, S.; Wu, J.; Du-Thumm, L.; Viana da Silva, C.L.; Roubicek, D.A.; Maria-Engler, S.S.; et al. Air Particulate Matter Induces Skin Barrier Dysfunction and Water Transport Alteration on a Reconstructed Human Epidermis Model. J. Investig. Dermatol. 2020, 140, 2343–2352.e3.
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 1578.
- Stefanovic, N.; Irvine, A.D.; Flohr, C. The Role of the Environment and Exposome in Atopic Dermatitis. Curr. Treat. Options Allergy 2021, 8, 222–241.
- Sarama, R.; Matharu, P.K.; Abduldaiem, Y.; Corrêa, M.P.; Gil, C.D.; Greco, K.V. In Vitro Disease Models for Understanding Psoriasis and Atopic Dermatitis. Front. Bioeng. Biotechnol. 2022, 10, 803218.
- Percoco, G.; Patatian, A.; Eudier, F.; Grisel, M.; Bader, T.; Lati, E.; Savary, G.; Picard, C.; Benech, P. Impact of Cigarette Smoke on Physical-chemical and Molecular Proprieties of Human Skin in an Ex Vivo Model. Exp. Dermatol. 2021, 30, 1610–1618.
- Ono, Y.; Torii, K.; Fritsche, E.; Shintani, Y.; Nishida, E.; Nakamura, M.; Shirakata, Y.; Haarmann-Stemmann, T.; Abel, J.; Krutmann, J.; et al. Role of the Aryl Hydrocarbon Receptor in Tobacco Smoke Extract-Induced Matrix Metalloproteinase-1 Expression. Exp. Dermatol. 2013, 22, 349–353.
- Hoskin, R.; Pambianchi, E.; Pecorelli, A.; Grace, M.; Therrien, J.-P.; Valacchi, G.; Lila, M.A. Novel Spray Dried Algae-Rosemary Particles Attenuate Pollution-Induced Skin Damage. Molecules 2021, 26, 3781.
- D’Agostini, F.; Balansky, R.; Pesce, C.; Fiallo, P.; Lubet, R.A.; Kelloff, G.J.; De Flora, S. Induction of Alopecia in Mice Exposed to Cigarette Smoke. Toxicol. Lett. 2000, 114, 117–123.
- Lecas, S.; Boursier, E.; Fitoussi, R.; Vié, K.; Momas, I.; Seta, N.; Achard, S. In Vitro Model Adapted to the Study of Skin Ageing Induced by Air Pollution. Toxicol. Lett. 2016, 259, 60–68.
- Langton, A.K.; Tsoureli-Nikita, E.; Merrick, H.; Zhao, X.; Antoniou, C.; Stratigos, A.; Akhtar, R.; Derby, B.; Sherratt, M.J.; Watson, R.E.B.; et al. The Systemic Influence of Chronic Smoking on Skin Structure and Mechanical Function. J. Pathol. 2020, 251, 420–428.
- Wedel, S.; Martic, I.; Hrapovic, N.; Fabre, S.; Madreiter-Sokolowski, C.T.; Haller, T.; Pierer, G.; Ploner, C.; Jansen-Dürr, P.; Cavinato, M. TBHP Treatment as a Model for Cellular Senescence and Pollution-Induced Skin Aging. Mech. Ageing Dev. 2020, 190, 111318.
- Petracca, B.; Nădăban, A.; Eeman, M.; Gooris, G.S.; Bouwstra, J.A. Effects of Ozone on Stratum Corneum Lipid Integrity and Assembly. Chem. Phys. Lipids 2021, 240, 105121.
- Valacchi, G.; Pagnin, E.; Corbacho, A.M.; Olano, E.; Davis, P.A.; Packer, L.; Cross, C.E. In Vivo Ozone Exposure Induces Antioxidant/Stress-Related Responses in Murine Lung and Skin. Free Radic. Biol. Med. 2004, 36, 673–681.
- Valacchi, G.; van der Vliet, A.; Schock, B.C.; Okamoto, T.; Obermuller-Jevic, U.; Cross, C.E.; Packer, L. Ozone Exposure Activates Oxidative Stress Responses in Murine Skin. Toxicology 2002, 179, 163–170.
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C Compound Mixtures Prevent Ozone-Induced Oxidative Damage in Human Keratinocytes as Initial Assessment of Pollution Protection. PLoS ONE 2015, 10, e0131097.
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161.
- Valacchi, G.; Pecorelli, A.; Belmonte, G.; Pambianchi, E.; Cervellati, F.; Lynch, S.; Krol, Y.; Oresajo, C. Protective Effects of Topical Vitamin C Compound Mixtures against Ozone-Induced Damage in Human Skin. J. Investig. Dermatol. 2017, 137, 1373–1375.
- Chavatte, L.; Juan, M.; Mounicou, S.; Leblanc Noblesse, E.; Pays, K.; Nizard, C.; Bulteau, A.-L. Elemental and Molecular Imaging of Human Full Thickness Skin after Exposure to Heavy Metals. Metallomics 2020, 12, 1555–1562.
More
