Bilberry (Vaccinium myrtillus L.) is one of the richest natural sources of anthocyanins which give berries their red/purple/blue coloration. Anthocyanins are powerful antioxidants and are reported to play an important role in the prevention of metabolic disease and CVD as well as cancer and other conditions.
- bilberry
- antioxidant
- anti-inflammatory
- cardiovascular disease
- hypoglycemic effect
- type 2 diabetes
1. Introduction
2. Chemical Structure, Distribution and Bioavailability of Anthocyanins
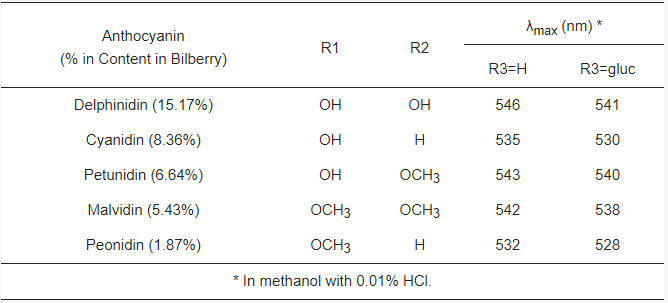
Anthocyanins are water-soluble polyphenolic vascular pigments that give berries their bright coloration [8,10][8][10]. The relative color of anthocyanins in aqueous solution is pH dependent. In acidic conditions, anthocyanins appear as red, but turn blue when the pH increases and finally become colorless at very high pH [19]. In terms of chemical structure, anthocyanins are glycosylated, polyhydroxy or polymethoxy derivatives of 2-phenylbenzopyrylium (flavylium cation) that contain two phenyl rings (A and B) separated by a hetero-cyclic (C) ring [20]. Anthocyanins usually contain a single glucoside unit but vary in the number of hydroxyl groups, the nature and number of sugars attached to the molecule, the position of the attachment, and the nature and number of aliphatic or aromatic acids attached to sugars in the molecule [16]. The main anthocyanins found in bilberry in decreasing contents are delphinidins (15.17%), cyanidins (8.36%), petunidins (6.64%), malvidins (5.43%) and peonidins (1.87%) [11,20][11][20] (Figure 1). Common sugars that attach to anthocyanins include glucose (Glu), galactose (Gal), arabinose (Ara), rutinose (Rut), rhamnose (Rham), and xylose (Xyl) and these sugars are bound as mono-, di-, or trisaccharide forms [21]. Anthocyanins have powerful antioxidant properties, and the content of anthocyanin directly correlates with the antioxidant activity of plants [11,22,23,24][11][22][23][24].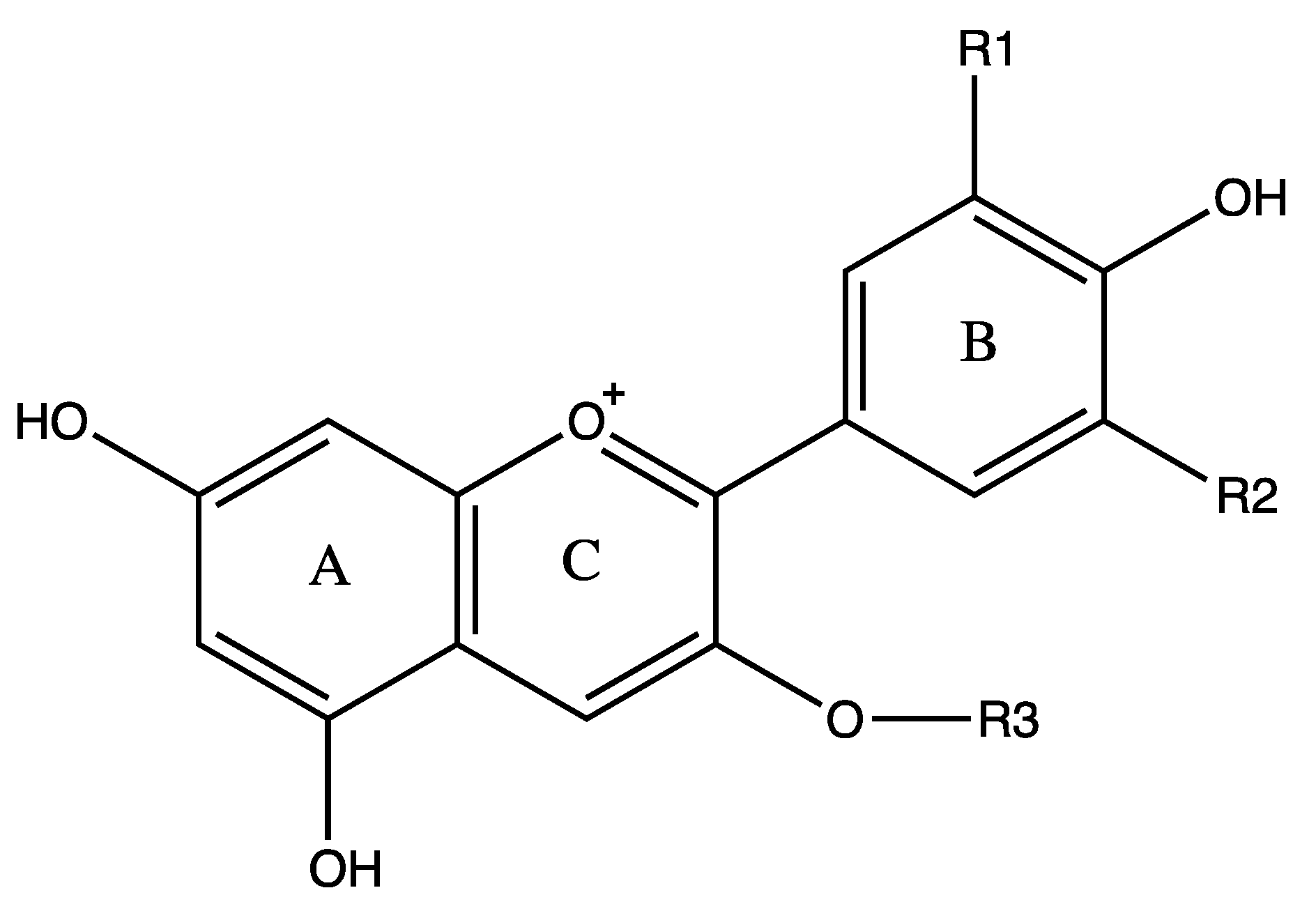
3. Beneficial Effects of Bilberries
Considerable attention has focused on the health benefits of dietary polyphenols, including anthocyanins. In vitro experiments, animal studies and clinical trials suggested that consumption of anthocyanins results in antioxidant, anti-inflammatory, anti-diabetic, anti-dyslipidemic and anti-hypertensive effects and the health benefits are associated with their potential antioxidant effect. Table 1 lists several of the important clinical studies that have investigated the health benefits of bilberry supplementation in healthy subjects or in subjects with increased CVD risk.|
Authors |
Type of Study |
Subjects |
Interventions |
Findings |
|---|---|---|---|---|
|
Antioxidant effect |
||||
|
Randomized controlled trial |
60 healthy volunteers |
100 g deep-frozen berries (bilberries, lingonberries, or blackcurrants) daily for 8 weeks; 240 g berries in postprandial study; or 500 g calcium gluconate |
Increased serum ascorbate, slight decrease in LDL oxidation, slight increase in serum antioxidant capacity in berry group; decreased LDL oxidation in postprandial study |
|
|
Duthie et al. [18] |
Randomized controlled trial |
20 healthy volunteers |
750 mL/day of cranberry juice (Ocean Spray Cranberry Select) or placebo drink (natural mineral water with strawberry flavor + sucrose (9 g/100mL)) for 2 weeks |
No effect on blood or cellular antioxidant status, lipid status, or oxidative DNA damage between groups |
|
Randomized controlled trial |
62 volunteers with increased risk of CVD |
330 mL/day bilberry juice (Corona Safteri, Rotvoll, Norway) or water for 4 weeks |
No effect on antioxidant status or oxidative stress |
|
|
Randomized controlled trial |
50 patients who were within 24 h of percutaneous coronary intervention |
Bilberry powder (40 g/d, equivalent to 480 g fresh bilberries) or no supplementation over 8 weeks |
Reduced total and LDL cholesterol compared to baseline; no difference in total and LDL cholesterol between groups |
|
|
Anti-inflammatory effect |
||||
|
Randomized controlled trial |
27 volunteers with features of metabolic syndrome |
400 g/day fresh bilberries or habitual diet for 8 weeks |
Reduced hsCRP, IL-6, IL-12, and LPS concentrations |
|
|
Randomized controlled trial |
62 volunteers with increased risk of CVD |
330 mL/day bilberry juice (Corona Safteri, Rotvoll, Norway) or water for 4 weeks |
Modulate NF-κB relatedinflammatory markers |
|
|
Randomized controlled trial |
120 healthy volunteers |
300 mg/day Medox (with purified anthocyanins isolated from bilberries and blackcurrant), or placebo (maltodextrin) capsules for 3 weeks |
Decreased NF-kB related pro-inflammatory chemokines, cytokines, and mediators of inflammatory responses |
|
|
Randomized placebo controlled, double-blinded trial |
150 hypercholesterolemia subjects |
Anthocyanins (320 mg/d) purified from bilberry and blackcurrant, or placebo for 24 weeks |
Decreased hsCRP, sVCAM-1, IL-1b and LDL cholesterol and increased HDL cholesterol |
|
|
Randomized controlled trial |
96 healthy volunteers |
Experimental diets either poor or rich in vegetables, berries and apple, and either richin linoleic acid or oleic acid for 6 weeks |
No effect on platelet activation or inflammation markers |
|
|
Hypoglycemic effect |
||||
|
Randomized placebo controlled, double-blinded cross-over study |
8 volunteers with T2DM controlled by diet and lifestyle |
0.47 g bilberry extract (36% (w/w) anthocyanins) capsule or placebo |
Decreased postprandial glycemia and insulin level |
|
|
Randomized placebo controlled, double-blinded trial |
120 overweight dyslipidemic subjects |
160 mg anthocyanins twice daily or placebo for 12 weeks |
No difference in glucose levels between groups |
|
|
Effects on dyslipidemia |
||||
|
Randomized placebo controlled, double-blinded trial |
120 overweight dyslipidemic subjects |
160 mg anthocyanins twice daily or placebo for 12 weeks |
Decreased LDL cholesterol and increased HDL cholesterol and inhibited CETP |
|
|
Randomized, placebo controlled, single-blind, trial |
71 volunteers with at least one CV risk factor |
100 g whole bilberries and 50 g lingonberries one every other day, and blackcurrant or strawberry purée and cold-pressed chokeberry and raspberry juice on alternative day, or placebo (sugar water, sweet semolina porridge, sweet rice porridge and marmalade sweets) for 8 weeks |
Reduced blood pressure, increased HDL cholesterol and prolonged PFA-100 CTs (CADP-CT) |
|
|
Randomized controlled, double-blinded trial |
150 hypercholesterolemic subjects |
320 mg/d anthocyanins purified from bilberry and blackcurrant, or placebo for 12 weeks |
Increased FMD, cGMP, and HDL cholesterol, and decreased serum sVCAM-1 and LDL cholesterol |
|
|
Randomized placebo-controlled, double-blind, parallel study |
122 hypercholesterolemic subjects |
320 mg/d anthocyanins purified from bilberry and blackcurrant, or placebo for 24 weeks |
Increased HDL cholesterol and decreased LDL cholesterol |
|
4. Adverse Effects of Bilberry
References
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010.
- Han, T.S.; Lean, M.E.J. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 13.
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143.
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412.
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005, 4, 5.
- Brownlee, M. The pathobiology of diabetic complications-A unifying mechanism. Diabetes 2005, 54, 1615–1625.
- Nath, S.D.; Habib, S.L.; Abboud, H.E. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005, 293, 2210–2211.
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P. Endothelial dysfunction, inflammation and diabetes. Rev. Endocr. Metab. Disord. 2004, 5, 189–197.
- Yang, Y.; Chan, S.W.; Hu, M.; Walden, R.; Tomlinson, B. Effects of some common food constituents on cardiovascular disease. ISRN Cardiol. 2011, 2011, 397136.
- Connor, A.M.; Luby, J.J.; Hancock, J.F.; Berkheimer, S.; Hanson, E.J. Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J. Agric. Food Chem. 2002, 50, 893–898.
- Upton, R. Bilberry fruit Vaccinium myrtillus L. In Standards of Analysis, Quality Control, and Therapeutics; American Herbal Pharmacopoeia and Therapeutic Compendium: Santa Cruz, CA, USA, 2001.
- Kowalczyk, E.; Krzesinski, P.; Kura, M.; Szmigiel, B.; Blaszczyk, J. Anthocyanins in medicine. Pol. J. Pharmacol. 2003, 55, 699–702.
- Chu, W.K.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011.
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48.
- Prior, R.L.; Wu, X.L. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028.
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933.
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629.
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1–21.
- Winefield, C.; Davies, K.; Gould, K. Anthocyanins: Biosynthesis, Functions, and Applications; Springer Science & Business Media: New York, NY, USA, 2009.
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520.
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683.
- Bagchi, D.; Roy, S.; Patel, V.; He, G.L.; Khanna, S.; Ojha, N.; Phillips, C.; Ghosh, S.; Bagchi, M.; Sen, C.K. Safety and whole-body antioxidant potential of a novel anthocyanin-rich formulation of edible berries. Mol. Cell. Biochem. 2006, 281, 197–209.
- Prior, R.L.; Cao, G.H.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693.
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333.
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043.
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food. Sci. Food Saf. 2013, 12, 483–508.
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120.
- Borges, G.; Roowi, S.; Rouanet, J.M.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. The bioavallability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007, 51, 714–725.
- Felgines, C.; Talavera, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Remesy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301.
- Felgines, C.; Texier, O.; Besson, C.; Fraisse, D.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are slightly bioavailable in rats. J. Nutr. 2002, 132, 1249–1253.
- Marczylo, T.H.; Cooke, D.; Brown, K.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother. Pharmacol. 2009, 64, 1261–1268.
- Sakakibara, H.; Ogawa, T.; Koyanagi, A.; Kobayashi, S.; Goda, T.; Kumazawa, S.; Kobayashi, H.; Shimoi, K. Distribution and excretion of bilberry anthocyanins in mice. J. Agric. Food Chem. 2009, 57, 7681–7686.
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Cao, G.H.; Muccitelli, H.U.; Sanchez-Moreno, C.; Prior, R.L. Anthocyanins are absorbed in glycated forms in elderly women: A pharmacokinetic study. Am. J. Clin. Nutr. 2001, 73, 920–926.
- Cao, G.; Russell, R.M.; Lischner, N.; Prior, R.L. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J. Nutr. 1998, 128, 2383–2390.
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737.
- Marniemi, J.; Hakala, P.; Maki, J.; Ahotupa, M. Partial resistance of low density lipoprotein to oxidation in vivo after increased intake of berries. Nutr. Metab. Carbiovasc. Dis. 2000, 10, 331–337.
- Karlsen, A.; Paur, I.; Bohn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kappa B related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355.
- Arevstrom, L.; Bergh, C.; Landberg, R.; Wu, H.X.; Rodriguez-Mateos, A.; Waldenborg, M.; Magnuson, A.; Blanc, S.; Frobert, O. Freeze-dried bilberry (Vaccinium myrtillus) dietary supplement improves walking distance and lipids after myocardial infarction: An open-label randomized clinical trial. Nutr. Res. 2019, 62, 13–22.
- Kolehmainen, M.; Mykkanen, O.; Kirjavainen, P.V.; Leppanen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimia, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510.
- Karlsen, A.; Retterstol, L.; Laake, P.; Paur, I.; Kjolsrud-Bohn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappa B activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954.
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Carbiovasc. Dis. 2013, 23, 843–849.
- Freese, R.; Vaarala, O.; Turpeinen, A.M.; Mutanen, M. No difference in platelet activation or inflammation markers after diets rich or poor in vegetables, berries and apple in healthy subjects. Eur. J. Nutr. 2004, 43, 175–182.
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, e22.
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.T.; Liu, J.; Mou, H.; Cao, L.; Ling, W.H. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492.
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331.
- Zhu, Y.N.; Xia, M.; Yang, Y.; Liu, F.Q.; Li, Z.X.; Hao, Y.T.; Mi, M.T.; Jin, T.R.; Ling, W.H. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533.
- Zhu, Y.N.; Huang, X.W.; Zhang, Y.H.; Wang, Y.; Liu, Y.; Sun, R.F.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569.
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohns Colitis 2013, 7, 271–279.
