Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Zhi Chen.
It is not paradoxical that what has been overlooked tends to be of great importance. Peroxisomes, the widely distributed organelles in the body, play irreplaceable roles in cellular metabolism, especially in fatty acid oxidation (FAO) and the generation and elimination of reactive oxygen species (ROS).
- Peroxisomal β-Oxidation
- Fatty acids
1. Introduction
Fatty acids (FAs) can be divided into four types according to the length of the carbon chain, namely short-chain fatty acids (containing 2–4 carbon atoms, SCFAs), medium-chain fatty acids (containing 6–12 carbon atoms, MCFAs), long-chain fatty acids (containing 13–18 carbon atoms, LCFAs) and very long-chain fatty acids (containing 20 or more carbon atoms, VLCFAs) [1]. In this Hereview, we will referin, focuses are to VLCFAs, including C20:0, C20:1, C22:0, and others. Any of these VLCFAs have important functions that cannot be substituted by LCFAs, such as skin barrier formation, retinal functions, resolution of inflammation, maintenance of myelin, sperm development and maturation, and liver homeostasis.
Most carbon saturated fatty acids (SFAs) are metabolized by β-oxidation in mitochondria. However, some specific FAs, such as unsaturated fatty acids (UFAs), branched-chain fatty acids (BCFAs), and VLCFAs, require different oxidation processes, including isomerization, alpha-oxidation (α-oxidation), omega-oxidation (ω-oxidation), and the oxidation process in peroxisomes. Similar to β-oxidation in mitochondria, four sequential reactions also occur in peroxisomal β-oxidation [2]. Despite similarities in the reactions, mitochondria and peroxisomes still have different catalytic proteins, electron transport chains, and orientations of metabolites, all of these suggest that research on mitochondria cannot be applied to peroxisomes (Figure 1).
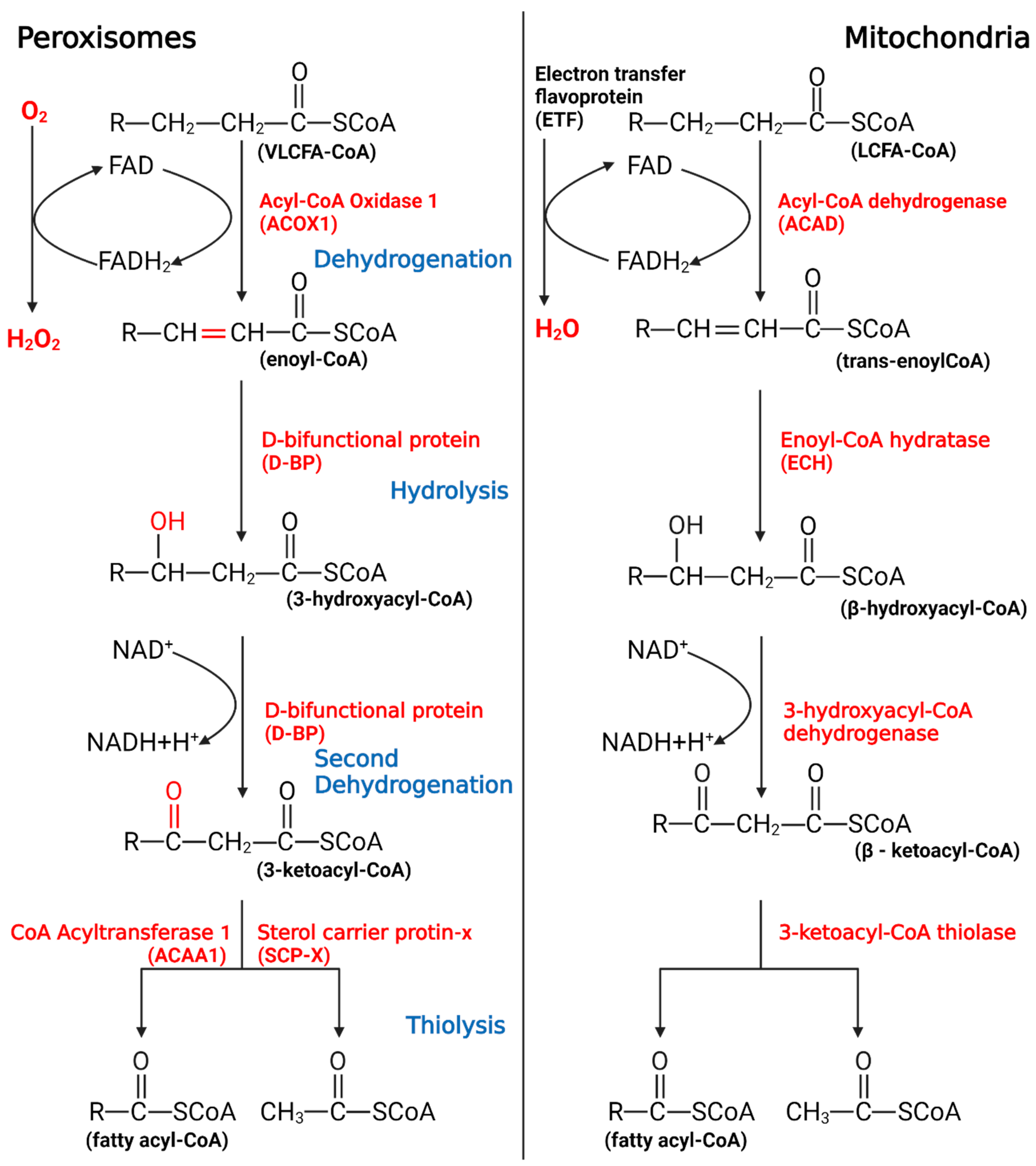
Figure 1.
The process of peroxisomal β-oxidation.
VLCFAs enter the peroxisome in the form of -CoA, start the β-oxidation from the first oxidation, undergo hydrolysis, begin the second oxidation, undergo thiolysis, and remove two C atoms. If VLCFAs are still present, they will enter the cycle again until the number of carbon chains is less than 18, after which the products will enter the mitochondria to continue metabolism.
While peroxisomal β-oxidation plays a role in fat metabolism, researchers habitually look for solutions in mitochondria when encountering problems associated with fat metabolism, especially cancer. Taking prostate cancer (PCa) as an example, multiple studies have demonstrated that the occurrence of PCa is related to free FAs and oxidative stress in the body [3], whereas most studies focus on regulating mitochondria to avoid or treat PCa; thus, peroxisomes have not been taken seriously yet. Interestingly, since 2015, certain studies have proposed to locate some relevant biomarkers in PCa cells peroxisome, such as monocarboxylate transporter 2 [4]. Some studies have also indicated that the expression of peroxisomal β-oxidation changes with PCa proliferation, and the rate of β-oxidation might affect the homeostasis of PCa cells [5]. By contrast, the specific mechanism of action between peroxisome and PCa and how to treat PCa through peroxisome remained unclear. It can be seen that peroxisome β-oxidation may also be the target scheme in many problems, but it is always overlooked. These days most studies focus on the interaction between peroxisome and mitochondria, whereas fewer studies have been done on its independent role. So, does it have research significance?
2. The Significance of Peroxisomal β-Oxidation
In fatty acid oxidation (FAO), the oxidation of VLCFAs is an aspect of peroxisome that differs from mitochondria. The mitochondrial β-oxidation pathway has long been considered to play a central role in lipid degradation [6[6][7],7], and any blockage of the oxidative pathway leads to increased lipid levels in tissues, yet the role of peroxisomes has been considered. Dysregulation of VLCFAs, essential components in the body, can lead to the occurrence of many diseases [8]. In humans, studies have shown that the accumulation of VLCFAs is the main cause of many neurological diseases, such as Alzheimer’s disease, multiple sclerosis, and dementia. In addition, studies of sexually transmitted diseases have found that VLCFAs are associated with ichthyosis, myopathy, and demyelination [9]. To be specific, VLCFAs accumulate in the plasma and tissue of patients, resulting in a fatal neurodegenerative phenotype, including childhood-onset cerebral adrenoleukodystrophy (CCALD) and adrenomyeloneuropathy (AMN) [10]. AMN is the milder phenotype characterized by a slowly progressive axonopathy. Thus, VLCFAs are not only an indispensable part of the body but also a substance that, if dysregulated in vivo, may result in strong toxicity.
In living organisms, free FAs, generally with low concentration, are mainly bound to fatty acid-binding proteins [11,12][11][12]. In this case, FAs in vivo are usually produced by the degradation of deposited fat and basically do not contain VLFCAs, and mitochondrial β-oxidation is the primary way of FAO. However, when the toxic VLCFAs enter the body or are in a free state, the peroxisome immediately activates the transport capacity through the transporter; in turn, acyl-CoA oxidase 1 (ACOX1) functions to ensure that peroxisomes can preferentially process VLCFAs that are not suitable for the internal environment. It is this timely processing mechanism that makes the intoxication caused by VLCFAs rare in the body. Of course, this may also be part of the reason why peroxisomal β-oxidation is easily overlooked.
Peroxisomal β-oxidation also plays an irreplaceable role in coping with oxidative stress. When the body is subjected to various harmful stimuli, highly active molecules such as ROS and reactive nitrogen species (RNS) generate excessive free radicals, and the oxidation degree exceeds the antioxidant capacity of cells to remove oxides. The oxidative and antioxidant systems are unbalanced, leading to tissue damage. This is related to the ratio of FADH2/NADH (F/N) (an electron transport chain involved in the transfer of free hydrogen ions and electrons) entering the electron transport chain. In short, the length of the FA carbon chain will affect the saturation and the F/N ratio, which in turn affects ROS production. If VLCFAs are metabolized in mitochondria, the ROS formed can cause severe oxidative stress in mitochondria. Special oxidation products generated during peroxisomal β-oxidation effectively reduced the occurrence of oxidative stress (Figure 2). In peroxisomes, the high-energy electrons stored in FADH2 are directly transferred from O2 to H2O2, and subsequently decomposed into H2O and O2. Therefore, the oxidation of VLCFAs in peroxisomes can reduce the amount of β-oxidation in mitochondria, thereby reducing the F/N ratio and ROS formation. Of course, peroxisomal β-oxidation inevitably causes ATP loss, and the energy carried by FADH2 is not used to synthesize ATP but is dissipated in the form of thermal energy. However, this heat loss is not a physiologically ineffective behavior, since studies have shown that peroxisomes accelerate the decomposition of FAs in BAT under cold stress conditions to help the body quickly adapt [13]. Therefore, peroxisomal β-oxidation is an important metabolic activity during nonshivering thermogenesis. Correspondingly, there is noise stimulation. In an extremely noisy environment, ROS production increases and causes oxidative damage, and peroxisomes can also regulate disorders caused by these stimuli through β-oxidation. Current research shows that this oxidative feedback regulation mechanism is activated by PEX5 [14].
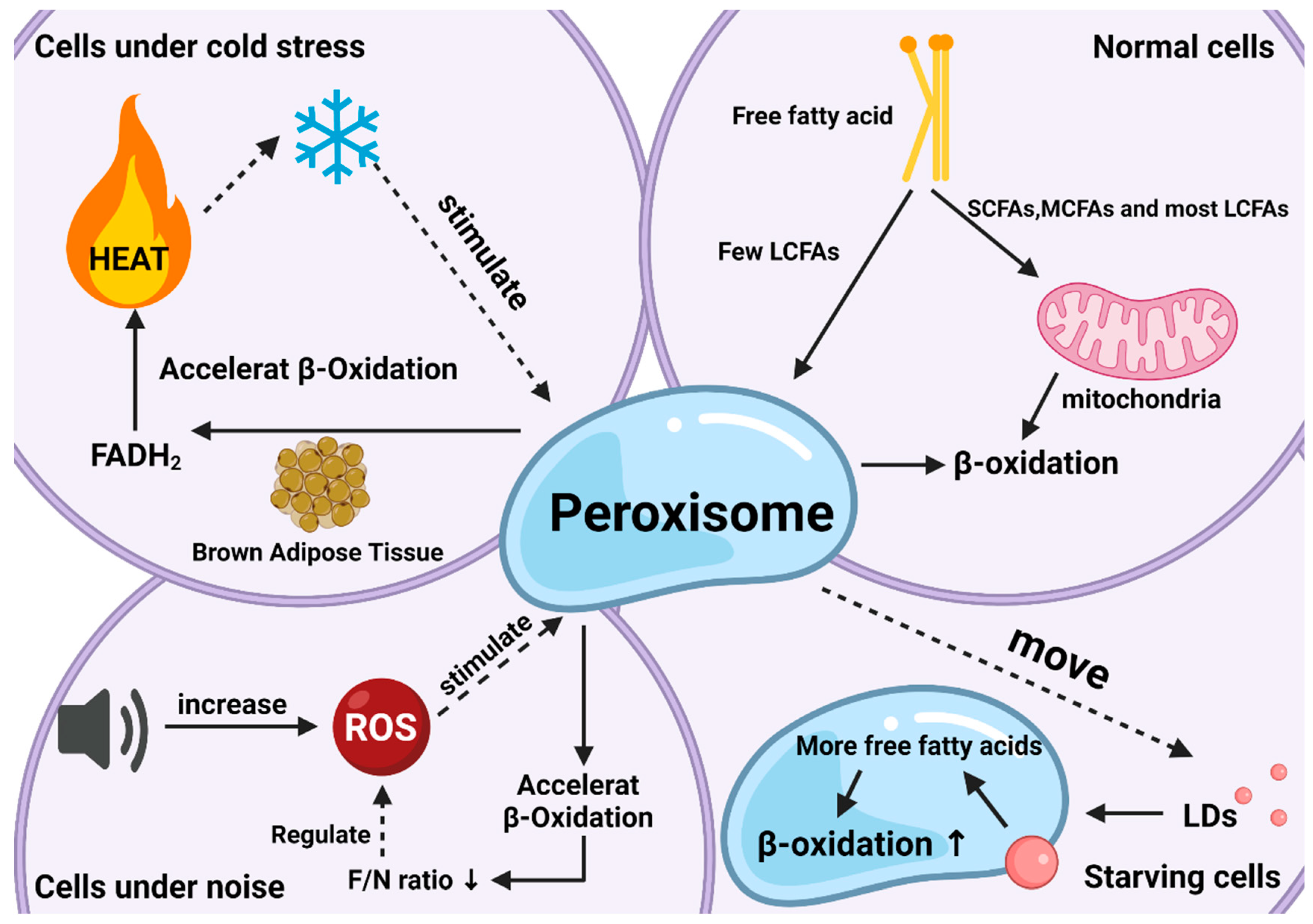
Figure 2. The roles of peroxisomal β-oxidation under various conditions. Responses and effects of peroxisomal β-oxidation in cells under normal (top right), starvation (bottom right), cold stress (top left), and noise (bottom left) conditions.
Another neglected effect is related to lipid droplets (LDs). LDs are lipid-storing organelles present in nearly all organisms, from bacteria to mammals, and their degradation provides metabolic energy for different cellular processes, such as membrane synthesis and molecular signaling [15]. Studies have shown that LDs and peroxisomes are generated at the same place in the endoplasmic reticulum with close subcellular localization after maturation, implying the possibility of interaction between the two organelles [16]. Indeed, researchers have found that when the body is starving, peroxisomes can move to and contact LDs with the help of kinesin KLFC3, and then transfer lipids from lipid droplets into the β-oxidation process more quickly to promote their degradation and maintain energy balance [17]. The latest research also authenticates this conclusion and gives a more specific explanation for “starvation” this condition arises to protect the body from ROS, since starvation increases fatty acid peroxidation as does the production of ROS [18].
Among mammals, peroxisomes also play an important role in ruminants, especially dairy cows. In other non-ruminant mammals, where FAO occurs mainly in mitochondria (76%), in ruminants, FAO occurs in mitochondria and peroxisomes (approximately 50% in each organelle) [19]. As dairy livestock are important, many studies have focused on the different lactation stages and butterfat percentage of dairy cows. Consumer choices today are based not only on the nutritional aspects of the food but also on products known to promote better health or prevent disease [20,21][20][21]. In this regard, the proportion of VLCFAs in milk is a concern. Dairy cows have a complete pathway for the synthesis and utilization of VLCFAs, through ELOVL protein synthesis and peroxisome utilization [22]. In actual production, high-producing dairy cows are subject to constant oxidative stress due to a high metabolic rate and physiological adaptation to intensive farming [23]. During the perinatal period of dairy cows, the body will undergo complex physiological changes, among which ketosis often occurs [24]. The occurrence of ketosis is often accompanied by fat deposition in the liver [25]. The essence is that after excess NEFAs (nonesterified fatty acid, fatty acids above C10, mainly VLCFAs) enter the liver, part of NEFAs enter the ketone body synthesis pathway to generate ketone bodies [26,27][26][27]. Study showed a greater level of ROS in mammary epithelial cells of ketotic cows, and greater oxidant indices, indicating increased oxidative stress status [28,29][28][29]. Although there is no direct research to prove that the occurrence of ketosis is related to peroxisomes, many studies have proved that factors related to peroxisomal β-oxidation are involved in the occurrence and control of the disease, such as PPARα, and AMPK, combined with the presence of VLCFAs, it we think this isas thought to be very possibly related to peroxisomes [30,31][30][31]. Also, many diseases in dairy cows can be attributed to oxidative stress, such as mastitis and breast edema [32,33,34,35][32][33][34][35]. Although some studies use extrinsic drugs to treat diseases from oxidative stress [36[36][37][38],37,38], it wauthors believed that the harm caused by oxidative stress can be alleviated by intrinsically regulating the rate of peroxisomal β-oxidation. Unfortunately, at present, few studies have linked peroxisomal β-oxidation to these diseases.
Overall, current research on peroxisomal β-oxidation has demonstrated its importance as a major factor in regulating lipid metabolism disorders in the internal environment and maintaining the balance of lipids and ROS. Unfortunately, most research on these functions has focused on understanding how they operate, and the current understanding of molecular-level mechanisms of functions remains limited.
References
- Chen, Z.; Lu, Q.; Liang, Y.; Cui, X.; Wang, X.; Mao, Y.; Yang, Z. Circ11103 Interacts with miR-128/PPARGC1A to Regulate Milk Fat Metabolism in Dairy Cows. J. Agric. Food Chem. 2021, 69, 4490–4500.
- Hiltunen, J.K.; Filppula, S.A.; Häyrinen, H.M.; Koivuranta, K.T.; Hakkola, E.H. Peroxisomal beta-oxidation of polyunsaturated fatty acids. Biochimie 1993, 75, 175–182.
- Zhou, X.; Mei, H.; Agee, J.; Brown, T.; Mao, J. Racial differences in distribution of fatty acids in prostate cancer and benign prostatic tissues. Lipids Health Dis. 2019, 18, 189.
- Valença, I.; Pértega-Gomes, N.; Vizcaino, J.R.; Henrique, R.M.; Lopes, C.; Baltazar, F.; Ribeiro, D. Localization of MCT2 at peroxisomes is associated with malignant transformation in prostate cancer. J. Cell. Mol. Med. 2015, 19, 723–733.
- Ha, X.; Wang, J.; Chen, K.; Deng, Y.; Zhang, X.; Feng, J.; Li, X.; Zhu, J.; Ma, Y.; Qiu, T.; et al. Free Fatty Acids Promote the Development of Prostate Cancer by Upregulating Peroxisome Proliferator-Activated Receptor Gamma. Cancer Manag. Res. 2020, 12, 1355–1369.
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta. Bioenerg. 2018, 1859, 940–950.
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702.
- Erdbrügger, P.; Fröhlich, F. The role of very long chain fatty acids in yeast physiology and human diseases. Biol. Chem. 2020, 402, 25–38.
- Mueller, N.; Sassa, T.; Morales-Gonzalez, S.; Schneider, J.; Salchow, D.J.; Seelow, D.; Knierim, E.; Stenzel, W.; Kihara, A.; Schuelke, M. De novo mutation in ELOVL1 causes ichthyosis, acanthosis nigricans, hypomyelination, spastic paraplegia, high frequency deafness and optic atrophy. J. Med. Genet. 2019, 56, 164–175.
- Hama, K.; Fujiwara, Y.; Takashima, S.; Hayashi, Y.; Yamashita, A.; Shimozawa, N.; Yokoyama, K. Hexacosenoyl-CoA is the most abundant very long-chain acyl-CoA in ATP binding cassette transporter D1-deficient cells. J. Lipid Res. 2020, 61, 523–536.
- Chen, Z.; Cao, X.; Lu, Q.; Zhou, J.; Wang, Y.; Wu, Y.; Mao, Y.; Xu, H.; Yang, Z. circ01592 regulates unsaturated fatty acid metabolism through adsorbing miR-218 in bovine mammary epithelial cells. Food Funct. 2021, 12, 12047–12058.
- Chen, Z.; Zhou, J.; Wang, M.; Liu, J.; Zhang, L.; Loor, J.J.; Liang, Y.; Wu, H.; Yang, Z. Circ09863 Regulates Unsaturated Fatty Acid Metabolism by Adsorbing miR-27a-3p in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2020, 68, 8589–8601.
- Park, H.; He, A.; Tan, M.; Johnson, J.M.; Dean, J.M.; Pietka, T.A.; Chen, Y.; Zhang, X.; Hsu, F.F.; Razani, B.; et al. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J. Clin. Investig. 2019, 129, 694–711.
- Defourny, J.; Aghaie, A.; Perfettini, I.; Avan, P.; Delmaghani, S.; Petit, C. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc. Natl. Acad. Sci. USA 2019, 116, 8010–8017.
- Zechner, R.; Madeo, F.; Kratky, D. Cytosolic lipolysis and lipophagy: Two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017, 18, 671–684.
- Joshi, A.S.; Nebenfuehr, B.; Choudhary, V.; Satpute-Krishnan, P.; Levine, T.P.; Golden, A.; Prinz, W.A. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat. Commun. 2018, 9, 2940.
- Kong, J.; Ji, Y.; Jeon, Y.G.; Han, J.S.; Han, K.H.; Lee, J.H.; Lee, G.; Jang, H.; Choe, S.S.; Baes, M.; et al. Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5. Nat. Commun. 2020, 11, 578.
- Karabiyik, C.; Vicinanza, M.; Son, S.M.; Rubinsztein, D.C. Glucose starvation induces autophagy via ULK1-mediated activation of PIKfyve in an AMPK-dependent manner. Dev. Cell 2021, 56, 1961–1975.e1965.
- Angeli, E.; Trionfini, V.; Gareis, N.C.; Matiller, V.; Huber, E.; Rey, F.; Salvetti, N.R.; Ortega, H.H.; Hein, G.J. Protein and gene expression of relevant enzymes and nuclear receptor of hepatic lipid metabolism in grazing dairy cattle during the transition period. Res. Vet. Sci. 2019, 123, 223–231.
- Harwood, W.S.; Drake, M.A. Validation of fluid milk consumer segments using qualitative multivariate analysis. J. Dairy Sci. 2020, 103, 10036–10047.
- Flowers, S.; McFadden, B.R.; Carr, C.C.; Mateescu, R.G. Consumer preferences for beef with improved nutrient profile1. J. Anim. Sci. 2019, 97, 4699–4709.
- Faria, O.A.C.; Kawamoto, T.S.; Dias, L.R.O.; Fidelis, A.A.G.; Leme, L.O.; Caixeta, F.M.C.; Gomes, A.; Sprícigo, J.F.W.; Dode, M.A.N. Maturation system affects lipid accumulation in bovine oocytes. Reprod. Fertil. Dev. 2021, 33, 372–380.
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14.
- Song, Y.; Loor, J.J.; Li, C.; Liang, Y.; Li, N.; Shu, X.; Yang, Y.; Feng, X.; Du, X.; Wang, Z.; et al. Enhanced mitochondrial dysfunction and oxidative stress in the mammary gland of cows with clinical ketosis. J. Dairy Sci. 2021, 104, 6909–6918.
- Cainzos, J.M.; Andreu-Vazquez, C.; Guadagnini, M.; Rijpert-Duvivier, A.; Duffield, T. A systematic review of the cost of ketosis in dairy cattle. J. Dairy Sci. 2022, 105, 6175–6195.
- Huang, Y.; Zhao, C.; Kong, Y.; Tan, P.; Liu, S.; Liu, Y.; Zeng, F.; Yuan, Y.; Zhao, B.; Wang, J. Elucidation of the mechanism of NEFA-induced PERK-eIF2α signaling pathway regulation of lipid metabolism in bovine hepatocytes. J. Steroid Biochem. Mol. Biol. 2021, 211, 105893.
- Mohsin, M.A.; Yu, H.; He, R.; Wang, P.; Gan, L.; Du, Y.; Huang, Y.; Abro, M.B.; Sohaib, S.; Pierzchala, M.; et al. Differentiation of Subclinical Ketosis and Liver Function Test Indices in Adipose Tissues Associated with Hyperketonemia in Postpartum Dairy Cattle. Front. Vet. Sci. 2021, 8, 796494.
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Sun, X.; Jia, H.; Zhao, C.; Xu, C. Adenosine 5’-monophosphate-activated protein kinase ameliorates bovine adipocyte oxidative stress by inducing antioxidant responses and autophagy. J. Dairy Sci. 2021, 104, 4516–4528.
- Li, Y.; Ding, H.; Liu, L.; Song, Y.; Du, X.; Feng, S.; Wang, X.; Li, X.; Wang, Z.; Li, X.; et al. Non-esterified Fatty Acid Induce Dairy Cow Hepatocytes Apoptosis via the Mitochondria-Mediated ROS-JNK/ERK Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 245.
- Xu, T.; Lu, X.; Arbab, A.A.I.; Wu, X.; Mao, Y.; Loor, J.J.; Yang, Z. Metformin acts to suppress β-hydroxybutyric acid-mediated inflammatory responses through activation of AMPK signaling in bovine hepatocytes. J. Anim. Sci. 2021, 99, skab153.
- Wu, Z.L.; Chen, S.Y.; Qin, C.; Jia, X.; Deng, F.; Wang, J.; Lai, S.J. Clinical Ketosis-Associated Alteration of Gene Expression in Holstein Cows. Genes 2020, 11, 219.
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986.
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenet. Chromatin 2018, 11, 60.
- Tabatabaee, N.; Heidarpour, M.; Khoramian, B. Milk metabolites, proteins and oxidative stress markers in dairy cows suffering from Staphylococcus aureus subclinical mastitis with or without spontaneous cure. J. Dairy Res. 2021, 88, 326–329.
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X.J. Selenium Deficiency Promotes Oxidative Stress-Induced Mastitis via Activating the NF-κB and MAPK Pathways in Dairy Cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726.
- Li, C.; Wang, Y.; Li, L.; Han, Z.; Mao, S.; Wang, G. Betaine protects against heat exposure-induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperones 2019, 24, 453–460.
- Ciampi, F.; Sordillo, L.M.; Gandy, J.C.; Caroprese, M.; Sevi, A.; Albenzio, M.; Santillo, A. Evaluation of natural plant extracts as antioxidants in a bovine in vitro model of oxidative stress. J. Dairy Sci. 2020, 103, 8938–8947.
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467.
More
