Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Hsin-Ying Chen and Version 2 by Beatrix Zheng.
Cancer stemness is proposed to be the main cause of metastasis and tumor relapse after conventional therapy due to the main properties of cancer stem cells. These include unlimited self-renewal, the low percentage in a cell population, asymmetric/symmetric cell division, and the hypothetical different nature for absorbing external substances. As the mechanism of how cancer stemness is maintained remains unknown, further investigation into the basic features of cancer stemness is required. Many articles demonstrated that glucose-regulated protein 78 (GRP78) plays a key role in cancer stemness, suggesting that this molecule is feasible for targeting cancer stem cells.
- GRP78
- cancer stem cells
- cancer stemness
- cell cycle
- cell division
1. Introduction
Cancer has been a high-ranked cause of death worldwide for many years [1], and there are no therapeutic strategies that effectively prevent metastasis and tumor relapse in all cancer types. With the disappointing outcomes of conventional therapeutic strategies [2], the novel explanations for the occurrence and progression of tumors are constantly being investigated for developing precise methods to eradicate tumors. Ever since the hypothesis of cancer stemness was proposed, the mechanisms of tumorigenesis and carcinogenesis have begun to lose their secret veil. Multiple cancer models demonstrated the existence of cancer stem cells [3][4][5][6][7][3,4,5,6,7], and the objective of eradicating tumors is believed to be achievable in the future. Due to the diverse background, concepts, and focus of different research groups, tumorigenic cells are called cancer stem cells or tumor-initiating cells, and different methods also have been utilized to investigate the various aspects of cancer stemness.
2. The Conventional Functions of GRP78, and Following Its Emerging Role in Cancer Stemness
GRP78 has an interesting history regarding its finding and naming. This molecule was originally observed and described in 1974 by Stone et al. For the purpose of generating a vaccine against the avian RNA tumor viruses, they described a plasma membrane protein of about 73 kDa that was upregulated in chick embryo fibroblasts transformed with Avian Sarcoma Viruses [8][71]. Until 1976, scientists had considered this 73 kDa membrane protein as a specific molecule related to virus transformation/infection. However, in 1977, Shiu et al. demonstrated that glucose starvation upregulated this 78 kDa membrane protein (analyzed by a better protein marker) in both immortalized and Rous virus-transformed chick embryo fibroblasts. Therefore, they concluded that this membrane protein was not related to viral transformation but was rather related to glucose regulation in cells, and thus it was named Glucose-Regulated Protein 78 (GRP78) [9][72]. Coincidentally, Haas et al. discovered an immunoglobulin heavy chain binding protein, named BiP, also weighing 78 kDa [10][73]. After cloning the cDNA of BiP, Haas et al. found that BiP belongs to the 70 kDa heat shock protein family [11][74] and participated in immunoglobulin chain synthesis and defective protein degradation in the endoplasmic reticulum [12][13][75,76]. Finally, Haas et al. clarified that BiP is GRP78 in a review publication [14][77]. Thereafter, it is known that GRP78 is not specific to glucose depletion nor virus infection. It is a resident chaperone in the endoplasmic reticulum whose physiological function is to facilitate normal protein production. GRP78 overexpression/upregulation is correlated with many stress/pathological conditions, such as hypoxia, radiation/ultraviolet exposure, immune diseases, low pH conditions, and most importantly, tumor malignancies [15][16][17][18][78,79,80,81].
There are many articles demonstrating a relationship between the expression levels of GRP78 protein and the severity of cancers and other diseases. Being the dominant resident chaperone in the endoplasmic reticulum and the main regulator functioning in the unfolded protein response in multiple physiological or pathological stress conditions, GRP78 has consistently been demonstrated to be upregulated, providing a protective effect and/or an essential function in multiple disease models. Total cellular levels of GRP78 received the most attention and investigation in the cancer models. GRP78 was found to be upregulated in prostate and head and neck cancers [18][19][81,82], and higher levels of GRP78 protein correlated with a poor prognosis of patients with these and lung cancers [19][20][21][82,83,84]. Importantly, hyper-expression of GRP78 in patients with head and neck cancers was demonstrated to be correlated with the malignancy levels of several oral diseases, as well as with the malignancy-free survival rates of precancerous oral diseases [21][84], highly supporting that cell surface GRP78 is a significant molecule for targeting cancer stem cells. GRP78 silencing also compromised the in vitro ability of migration and invasion, as well as in vivo tumor growth of head and neck cancer cells [18][22][51,81]. GRP78 downregulation enhanced the sensitivity to chemotherapy drugs of multiple types of cancer cells and tumor endothelial cells, including head and neck, breast, lung, colon, glioma, and bladder cancers [23][24][25][26][27][28][29][30][62,85,86,87,88,89,90,91]. Compared to the tissues of patients with benign ovarian tumors, malignant ovarian cancers have upregulated expression of GRP78 mRNA [31][92]. GRP78 was also studied in diseases other than cancer models. In a cardiac hypertension model, GRP78 protein expression was increased in cardiomyocytes after high blood pressure was induced in mice, and exogenous overexpression of GRP78 in cardiomyocytes enhanced hypertrophic growth of cardiomyocytes via the activation of GATA-Binding Protein 4, intensifying the level of cardiac vessel hypertension [32][93]. In the research project on type 2 diabetes mellitus in a Chinese population, GRP78 was detected in the circulating blood of these patients and circulating GRP78 also correlated with the severity of this kidney disease [33][94].
GRP78 expression on the cell surface of human rhabdomyosarcoma cells treated with thapsigargin and GRP78 autoantibody detected in normal human peripheral blood [34][95] suggested a potential signaling role of this molecule. GRP78 autoantibodies purified from the serum of prostate cancer patients exhibited pro-proliferative effects and increased intracellular calcium levels in cell lines of prostate cancer and melanoma; this autoantibody was shown to specifically recognize a tertiary structural motif mimicking an epitope in GRP78 [35][96]. Thereafter, cell surface GRP78 was hypothesized to function as an oncogenic signaling receptor, and the ligands of this hypothetic signaling receptor and the signaling outcomes have been extensively explored. The potential molecules associated with or binding to GRP78 were reported by multiple research groups, yet different ligand associations with cell surface GRP78 result in different signaling outcomes. For example, α2-macroglobulin [36][97] and Cripto [37][98] were shown to associate with cell surface GRP78 in prostate cancer cells and promoted signaling pathways of proliferation, metastasis, and tumor growth. When Par-4, TRAIL (tumor necrosis factor related apoptosis inducing ligand) [38][99] and recombinant Kringle 5 of human plasminogen [39][100] associated with cell surface GRP78 in prostate cancer, endothelial, and glioma cells, apoptosis was induced. GRP78 was reported as an autoantigen for B and T cells in rheumatoid arthritis patients [40][41][101,102]. Entry into the host cells of several virus strains, including Coxsackie virus A9 [42][103], Borna disease virus [43][104], severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, which causes COVID-19) [44][105], depended on GRP78 expressed on the cell surface of the host cells. Although it could be certain that GRP78 plays important roles in various cellular events, it was also questionable why a resident chaperone expressed in the endoplasmic reticulum in most of the somatic cells was reported to function as a signaling receptor in another subcellular compartment.
The molecules associated with cell surface GRP78 were never comprehensively and systematically investigated before Chen et al. reported their findings. Using the newly designed quantitative mass spectrometry platform, they detected and verified multiple endogenous interactome candidates of cell surface GRP78 and intracellular GRP78 in head and neck cancer cells [7]. There are also other articles reporting other molecules associated with cell surface GRP78 or intracellular GRP78 [17][18][19][20][21][80,81,82,83,84]. Collectively, these sound demonstrations consolidate that GRP78 still functions as a chaperone in the plasma membrane compartment. If the endogenous interactome of GRP78 in different subcellular compartments of other cancer models can be identified by the quantitative platform of mass spectrometry, not only will it provide the molecular machinery of malignancy phenotypes, but it will also provide detailed molecular profiles of cancer stemness due to GRP78’s roles in cancer stemness discussed below.
The previous findings of GRP78 led to the suggestion that GRP78 plays a role in cancer stemness. GRP78 overexpression in Chinese Hamster Ovary cells increased their resistance to Etoposide treatment [24][85]. Since Etoposide hampers the function of DNA polymerase, and cell division pattern and frequency are the characteristics within the scope of cancer stemness, this finding is the first to show that GRP78 can protect somatic stem cells from apoptosis. This is also an indirect suggestion that GRP78 may have functions in cancer stemness because of the possibility of the tumorigenic origin of somatic stem cells. Then, Wu et al. were the first to demonstrate the possibility of cell surface GRP78 serving as a cancer stem cell marker. They showed that the head and neck cancer cell population of cell surface GRP78hi (csGRP78hi) had better tumorigenesis capability than that of csGRP78lo in the mouse xenograft model determined by serial dilution of the transplanted cell numbers [22][51], which is one of the traditional methods to evaluate the occurrence, or the level, of cancer stemness. Since serial dilution of the cell number of sorted head and neck cancer cells based on the cell surface GRP78 level has a direct influence on xenograft tumorigenesis in a mouse model, cell surface GRP78 is hypothesized to serve a direct role in cancer stemness. Therefore, Chen et al. further investigated the influence of the expression levels of csGRP78 on other hypothetical properties of cancer stemness [7], including cell division pattern and cell cycle phase profile since asymmetric cell division is considered as a sign of differentiation in cell fate determination [45][106], and pluripotency state of embryonic stem cells was shown to be maintained in the cell cycle S and G2 phases [46][107]. They found that the percentages of csGRP78hi head and neck cancer cells in the G2/M cell cycle phase were more than 9-fold higher than that of csGRP78lo in the G1 cell cycle phase. By using the flow cytometry-based cell division assay developed by Chen et al., head and neck cancer cells expressing Progranulin on their cell surface exclusively perform symmetric cell division, while those expressing cell surface GRP78 perform both symmetric and asymmetric cell division. Meanwhile, GRP78 silencing downregulated multiple stemness-related markers. This investigation demonstrates that GRP78 is a chaperone related to cancer stemness maintenance in head and neck cancer cells (Figure 12), considering that multiple interactome molecules to csGRP78 were detected [7]. Furthermore, Chen et al. noticed that there was a distinct cell population expressing an ultra-high level of csGRP78 (Figure 23) in the symmetrically divided csGRP78+ cells in three head and neck cancer cell lines (The data presented in this researchtudy are available in Gate R3 of Figure 5B,D,F of reference [7]), but it is unknown whether these distinct cell populations possess cancer stemness of the highest rank of the hierarchy.
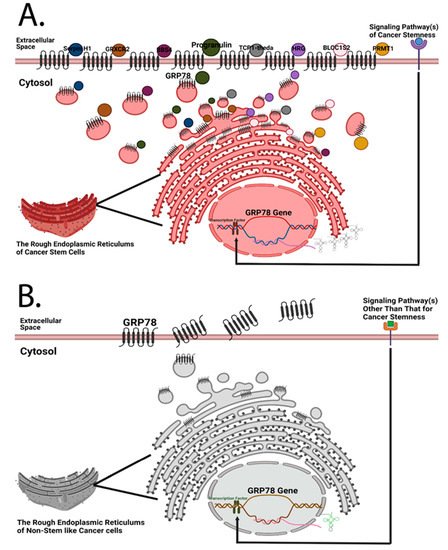
Figure 12. Both cancer stem cells and non-stem-like cancer cells are hypothesized to express cell surface GRP78, but the amounts of cell surface GRP78 and its interactome of these two types of cells are different. (A) Previously, Chen et al. designed a quantitative mass spectrometry platform to detect the interactome of cell surface GRP78 in head and neck cancer cells [7]. Given that GRP78 silencing has a regulatory influence on stemness-related markers [7], it is logical to hypothesize that cancer stem cells possess a more diverse GRP78 interactome than non-stem-like cancer cells; and that cancer cells expressing a higher amount of cell surface GRP78 may possess the highest hierarchical rank of cancer stemness. (B) Due to the higher proliferation rate of cancer cells, an increase in plasma membrane synthesis in the endoplasmic reticulum is required, resulting in the translocation of resident chaperone GRP78 to the cell surface. Under this circumstance, non-stem-like cancer cells may express a certain amount of cell surface GRP78 even if there are no plasma membrane-bound proteins that need to be chaperoned to the subcellular compartment of the plasma membrane. GRP78 is likely secreted afterwards.
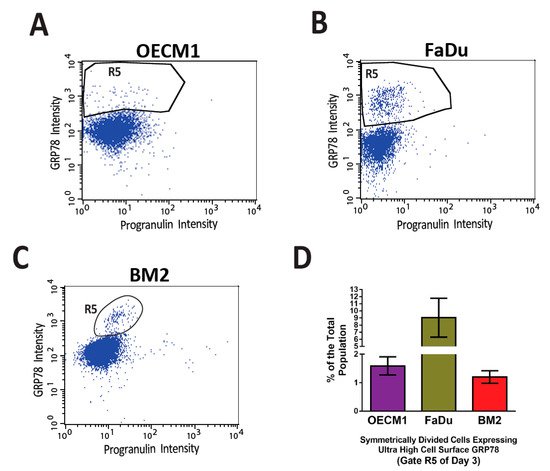
Figure 23. Distinct small populations of symmetrically divided cancer cells expressing ultra-high levels of cell surface GRP78 were consistently observed in the flow cytometry-based cell division assay. In a previously published article by Chen et al. [7], a high throughput flow cytometry-based cell division assay was developed to characterize the profile of asymmetric/symmetric cell divisions in the heterogeneous cell populations of three head and neck cancer cell lines, OECM1 (A), FaDu (B), and BM2 (C). In the three cancer cell lines that went through symmetric cell division (Gate R3 of Figure 5B,D,F of reference [7]), distinct cell populations expressed ultra-high levels of cell surface GRP78 (the R5 gates) consistently above the levels of major csGRP78 positive populations. (D) The quantification of the R5 gates of the three head and neck cancer cell lines. The percentages of R5 gates are 1–9% of the total cell populations.
Besides the head and neck cancer model, the potential function of GRP78 in cancer stemness is also reported in other cancer models. Exogenous expression of GRP78 in MDA-MB-231 cells increased the percentage of cell population expressing CD44+/CD24−, and cGRP78+ MCF7 cells exhibited better in vitro and in vivo tumorigenesis than the total unsorted and the CD44+/CD24− population [47][108]. GRP78 silencing significantly decreased the in vivo survival of glioma stem cells after ionizing radiation [48][109]. Being chaperoned by csGRP78 for lysosomal degradation, BACE2 silencing suppressed in vitro and in vivo tumorigenesis capability of glioma stem cells, demonstrating that csGRP78 engages in the regulation of cancer stemness in glioma [49][110]. Interestingly, using a CRISPR knockout system, GRP78 was shown to prime non-small lung adenocarcinoma cells for cell cycle re-entry after Cisplatin treatment, allowing cancer cells to escape the fate of senescence [50][111]. How cancer stem cells regulate cell cycle progression and initiate the entry or arrest is proposed to be a key cellular event of cell stemness maintenance [7]. The mechanism of how GRP78 contributes to cancer stemness needs to be investigated for precise targeting of cancer stem cells. Several cell-signaling pathways were suggested to be responsible for the regulation of self-renewal in cancer stem cells [51][112]. Therefore, verifying whether GRP78 regulates these pathways, or vice versa, will be valuable for understanding the mechanism of cancer stemness maintenance.
