Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 3 by Conner Chen.
The term nanomedicine indicates a specific targeted treatment, which can enhance the delivery of targeted drugs and their bioavailability, as well as reduce the associated toxicity or side-effects and costs, using nanoparticles (NPs). Thus, they constitute specialized carriers with the potential to facilitate the delivery of drugs and efficient molecular targets into desired tissues, such as the endothelium.
- endothelium
- nanomedicine
1. Innovative Technologies for Targeted Endothelium Treatments: Nanomedicine and Its Benefits and Limitations
The term nanomedicine indicates a specific targeted treatment, which can enhance the delivery of targeted drugs (i.e., natural antioxidant compounds or pharmacological drugs) and their bioavailability, as well as reduce the associated toxicity or side-effects and costs, using nanoparticles (NPs) [1]. NPs comprise liposomes, niosomes, polymers, lipid and organic polymer hybrids and precursors, carbon nanotubes, quantum dots, metals, and metal oxides (see Figure 1).
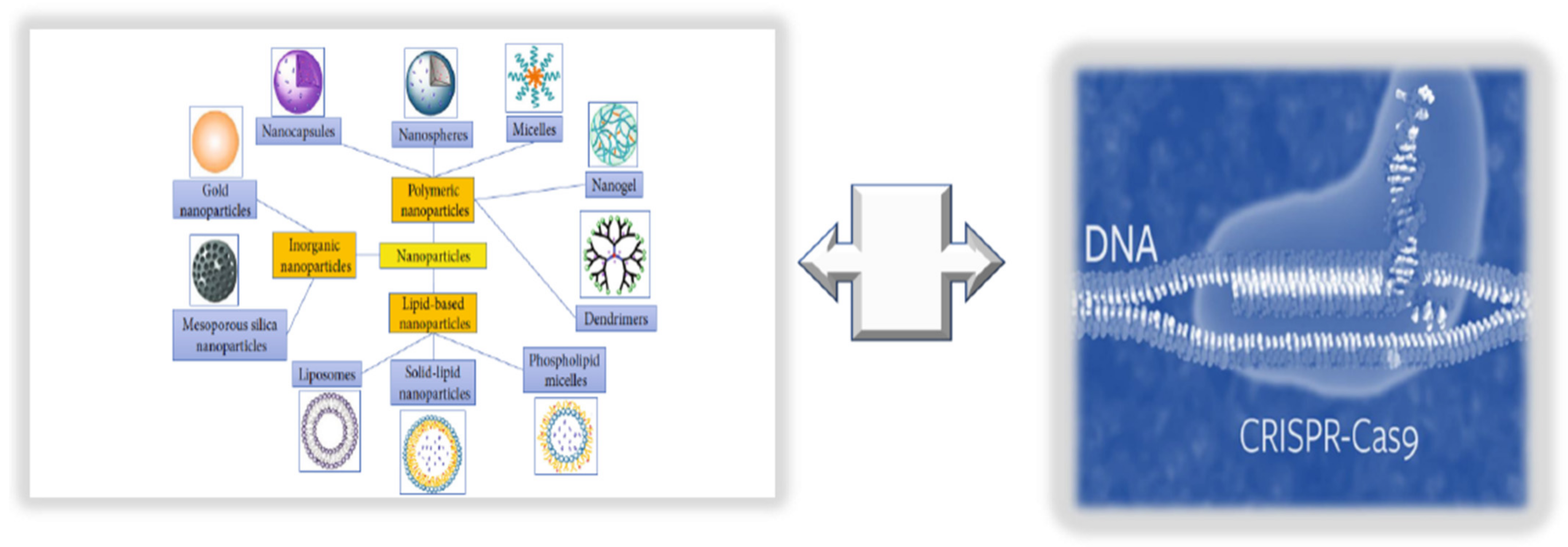
Figure 1. Innovative technologies for targeted endothelium treatments: nanomedicine + CRISPR/Cas9 editing.
Thus, they constitute specialized carriers with the potential to facilitate the delivery of drugs and efficient molecular targets into desired tissues, such as the endothelium. Furthermore, NPs show another benefit in improving the effectiveness of therapeutic compounds, i.e., by decreasing their toxicity or side-effects. To this aim, NPs feature two aspects: (1) a versatile preparation method and a structure that allows encapsulating therapeutic agents, and (2) a functional surface able to interact with antibodies, aptamers, small molecules, etc., to further facilitate the delivery of therapeutics or diagnostics to pathological sites of the endothelium [1][2]. Accordingly, the linking of NP surfaces with molecular fractions mimicking natural ligands can provide selective targeting and precise delivery of therapeutics to a diseased tissue. Nevertheless, only a small percentage of these NPs can successfully accumulate in the selected tissues. For example, Wilhelm and coworkers [3] showed that fewer than 1% of nanoparticles manage to accomplish their role, specifically related to tumoral tissues. The reasons are diverse and mostly related to multiple physiological barriers and the high degree of stochasticity involved in NP activity. Accordingly, a high percentage of NPs are phagocytized by the mononuclear phagocytic system (MPS), while some remain physically “entrapped” in the sinusoids of the liver and others are taken up by hepatocytes and Kupffer cells [1]. In addition, NPs are incapable of adequately negotiating other biological barriers. This has hampered their clinical translation. Consequently, innovative biomimetic strategies [1] have been developed. They are mainly based on two approaches: (1) top-down approaches, including the bioengineering of pathogens (bacteria and viruses) or cells (leukocytes, erythrocytes, platelets, and stem cells) [4], or (2) bottom-up approaches, such as conjugating NP surfaces with analogs of bioactive molecules that bind CAMs and selectins [5][6] or coating synthetic particles with cell membranes, as reported by Parodi and coworkers [7]. Additional approaches include poly(lactic-co-glycolic acid) (PLGA) NPs coated with a platelet cell membrane that preferentially bind to the denuded artery in a rat model of coronary restenosis [5]. Other biomimetic NPs exhibit endogenous surface molecules, such as low-density lipoprotein (LDL) and HDL, which have been used for incorporating nucleic material and delivering both therapeutic and diagnostic molecules [8].
Another important issue is the assessment of NPs’ biodistribution following in vivo administration in animals and humans [9][10]. This constitutes a great challenge despite the large range of techniques available for detecting nanoparticle biodistribution, including histology, electron microscopy, liquid scintillation counting (LSC), indirect measurement of drug concentrations, in vivo optical imaging, computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine imaging.
However, a biomimetic innovative approach has been utilized to improve the bioavailability, stability, and consequent advantageous actions of curcumin on endothelial related diseases [10]. Similarly, pH and ROS dual-responsive NPs fabricated by incorporating pH- and ROS-responsive cyclodextrin materials with resveratrol were utilized as an efficient and secure nanoplatform for therapeutic delivery to areas of vascular inflammation in the presence of acidosis and oxidative stress [11][12]. Cho and co-workers also used biomimetic NPs with synthetic HDL (liposomal formulation with dimyristoyl phosphatidylcholine (DPMC)), demonstrating that they could significantly enhance the blood HDL levels, as well as reduce the plaque development in an animal study [13]. In another study, using a murine model of inflammation, the researchers targeted E-selectins by conjugating anti-E-selectin monoclonal antibodies to ultra-small-SPION in vivo for imaging of the endothelium to detect vascular inflammation [14]
2. Consideration of NPs as New Endothelium Treatment Strategy
Currently, newer treatment strategies, with reduced risks of evocating adverse effects, are being investigated, among which nanomedicine shows a more targeted specificity and efficacy. Thanks to the discovery of various endothelium-targeting molecules, efforts to design NP treatments targeting specific endothelium sites and ligands using appropriate nano-carriers are increasing. However, there are several challenges, including the shear stress under continuous flow, the variable size of arteries and their marginalization from the bloodstream, and the elimination of NPs by macrophages, which affect NP internalization in the endothelium. Consequently, numerous delivery strategies have been developed to improve the adequate transport of medication into specific endothelial regions, as mentioned above, including the production of biomimetic NPs. However, the number of translational studies is inadequate for elucidating the safety and efficacy of such NPs, and their delivery strategies remain a challenge. To progress to clinical application, NPs should interact with and internalize in the endothelium effectively, and they should be proven to be nontoxic, more successful than existing options, easy to use, and cost- and design-effective. Nevertheless, significant benchwork associated with an increased number of clinical studies may provide the necessary hope for treating endothelium dysfunction with nanotechnology [15].
3. Integrating NPs with CRISPR/Cas9 Editing and Base Primers
In the case of the endothelium, NP application shows several limitations, as largely stressed above. While developing such treatments, it is imperative to consider the high degree of endothelium heterogeneity and complexity, owing to its diverse morphology, location, and function shown in various body tissues. Endothelial alterations are directly or indirectly involved in a wide variety of human disorders, encompassing several mechanisms and pathways (including inflammation), and they are caused by and promote the progression of endothelial dysfunction. This can severely affect other cellular types, in physiological or pathological conditions, impacting the function of organs and systems, as well as physiological processes, such as regeneration, repair, cardiovascular genesis, neurogenesis, and cardiovascular and neural survival. This is significantly related to the altered gene expression of cells linked or not to genetic variants and/or genomic modifications. Consequently, it is hypothesized that a strong genetic editing technique might be a solution, as well as the integration of gene-editing techniques with nanotechnology. Accordingly, the clustered regularly interspaced short palindromic repeats-associated protein 9 (CRISPR/Cas9) system and techniques to chemically modify RNAs are also being incorporated in NPs to study human pathologies and/or examine potential disease correction through restoration [16][17]. The CRISPR/Cas9 system is significantly utilized for both editing the genome of zygotes, thereby generating genetically modified animal species, and treating human diseases. In fact, the development of safe and efficient treatments is becoming a reality by delivering the CRISPR/Cas9 system into body tissues and targeting specific cells [18][19]. A delivery system with optimal in vivo genome-editing efficacy is based on the use of the recombinant adeno-associated virus (AAV), but it can cause adverse effects. Thus, lipid NPs represent an alternative. For example, lipid NPs were used to target macrophages in mice using the CRISPR/Cas9 system, although a reduced genome-editing percentage (20%) was obtained. This suggests that it is a challenge to induce robust genome editing in the targeted endothelium. However, hope can be derived from a recent study conducted by the Zhang group on ECs obtained from the lung, heart, aorta, and peripheral vessels of adult mice. Specifically, Zhang and coworkers [20] developed poly(ethylene glycol) methyl ether-block-poly(lactide-co-glycolide) (PEG-b-PLGA; PP)-based NPs with excellent biodistribution for vascular delivery and demonstrated that the polyethyleneimine (PEI)-formulated PP NP-mediated delivery of the all-in-one CRISPR plasmid DNA expressing Cas9 under the control of the human CDH5 promoter and a guide RNA (gRNA) driven by the U6 promoter determined highly efficient genome editing in ECs of various vascular locations with a single administration. A reduction in protein expression of about 80% was evidenced in ECs. The potential demonstrated by such an approach could allow efficiently modulating or eliminating the expression of genes encoding proteins strongly associated with phenotypic changes related to the development of endothelium dysfunction and its related pathologies [20]. Today, several new CRISPR-based modalities have been developed that are capable of facilitating gene editing without the requirement of a DNA double-strand break (DSB), including base editors, prime editors, and RNA-targeting CRISPR-associated protein (Cas)13 effectors [21]. These modalities can potentially be integrated with NPs. However, this will require completely optimizing individual base editor proteins for specific therapeutic targets to guarantee maximally efficient formation of the on-target product, as well as minimize potentially counterproductive nontarget editing outcomes. In addition to developing methods to further refine the capabilities of these increasingly sophisticated gene-editing machines, special attention must be given to their delivery. Thus, further studies are needed.
References
- Adamczyk, Z.; Morga, M.; Nattich-Rak, M.; Sadowska, M. Nanoparticle and bioparticle deposition kinetics. Adv. Colloid Interface Sci. 2022, 302, 102630.
- Chacko, A.M.; Hood, E.D.; Zern, B.J.; Muzykantov, V.R. Targeted nano-carriers for imaging and therapy of vascular inflammation. Curr. Opin. Colloid Interface Sci. 2011, 16, 215–227.
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014.
- Yoo, J.W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bio-engineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535.
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121.
- Robbins, G.P.; Saunders, R.L.; Haun, J.B.; Rawson, J.; Therien, M.J.; Hammer, D.A. Tunable leuko-polymersomes that adhere specifically to in-flammatory markers. Langmuir 2010, 26, 14089–14096.
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68.
- McMahon, K.M.; Mutharasan, R.K.; Tripathy, S.; Veliceasa, D.; Bobeica, M.; Shumaker, D.K.; Luthi, A.J.; Helfand, B.T.; Ardehali, H.; Mirkin, C.A.; et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011, 11, 1208–1214.
- Taneja, G.; Sud, A.; Pendse, N.; Panigrahi, B.; Kumar, A.; Sharma, A.K. Nano-Medicine and Vascular Endothelial Dysfunction: Options and Delivery Strategies. Cardiovasc. Toxicol. 2019, 19, 1–12.
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 3149223.
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 2019, 230, 119605.
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.-S.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844.
- Cho, B.H.; Park, J.R.; Nakamura, M.T.; Odintsov, B.M.; Wallig, M.A.; Chung, B.H. Synthetic dimyristoylphosphatidylcholine liposomes assimilating into high-density lipoprotein promote regression of atherosclerotic lesions in cholesterol-fed rab-bits. Exp. Biol. Med. 2010, 235, 1194–1203.
- Greene, J.A.; Loscalzo, J. Putting the Patient Back Together—Social Medicine, Network Medicine, and the Limits of Reductionism. N. Engl. J. Med. 2017, 377, 2493–2499.
- Pillai, S.C.; Borah, A.; Jacob, E.M.; Kumar, D.S. Nanotechnological approach to delivering nutraceuticals as promising drug candidates for the treatment of atherosclerosis. Drug Deliv. 2021, 28, 550–568.
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823.
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096.
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 2015, 21, 121–131.
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042.
- Zhang, X.; Jin, H.; Huang, X.; Chaurasiya, B.; Dong, D.; Shanley, T.P.; Zhao, Y.-Y. Robust genome editing in adult vascular endothelium by nanoparticle delivery of CRISPR-Cas9 plasmid DNA. Cell Rep. 2022, 38, 110196.
- Botto, C.; Dalkara, D.; El-Amraoui, A. Progress in Gene Editing Tools and Their Potential for Correcting Mutations Underlying Hearing and Vision Loss. Front. Genome Ed. 2021, 3, 737632.
More
