Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Zhang Wei and Version 4 by Sirius Huang.
Liquid crystal elastomers (LCEs) are programmable deformable materials that can respond to physical fields such as light, heat, and electricity. Photothermal-driven LCE has the advantages of accuracy and remote control and avoids the requirement of high photon energy for photochemistry. An indispensable part of photothermally driven LCEs materials are photothermal materials.
- liquid crystal elastomer
- smart material
- photothermal-driven liquid crystal elastomers
1. Liquid Crystal Elastomers
Since Finkelmann et al. first reported liquid crystal elastomers in 1981, liquid crystal elastomers (LCEs) have under-gone tremendous development in both material chemistry and processing, becoming a class of materials with special properties [1][11]. Unlike liquid crystal polymers that are not crosslinked and liquid crystal networks that are highly crosslinked, low-crosslinked liquid crystal polymer networks (Figure 1a) with anisotropic polymer chains are known as LCEs [2][4]. Due to the low degree of crosslinking, anisotropic polymer chains can temporarily become isotropic when heated beyond the nematic-to-isotropic phase transition temperature (TNI), resulting in contraction along the alignment (Figure 1b). At the same time, the degree of order in the LCEs is reduced with the loss of anisotropic chain conformation. The presence of the polymer network ensures that the LCE can obtain the original orientation when the temperature is lower than TNI. This determines that the LCE undergoes reversible anisotropic deformation when the temperature changes. In fact, different application scenarios require LCEs that possess different orientations and physical properties, which are affected by the synthesis and alignment of LCEs. In general, the synthetic routes can be summarized into two groups: two-step crosslinking and one-step crosslinking. For the two-step cross-linking route, the LCE can be mechanically oriented because of the presence of a partially cross-linked polymer network. In the one-step crosslinking route, low molecular weight monomers are directly polymerized to form LCEs. Due to the low viscosity of the precursors, the LCEs prepared by one-step cross-linking are suitable for surface-induced orientation.
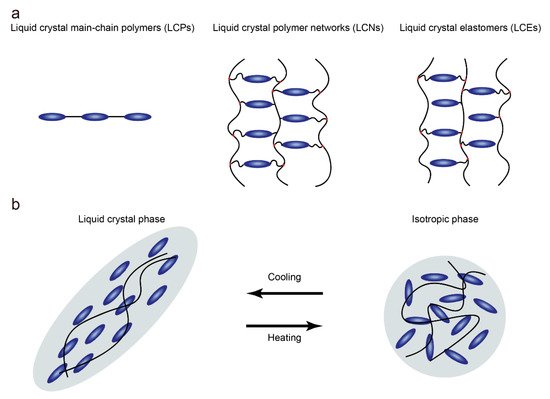
Figure 1. (a) Schematic of liquid crystal polymers, liquid crystal polymer networks and liquid crystal elastomers; (b) Schematic illustration of the contraction along the alignment of LCEs when heated beyond TNI.
1.1. Two-Step Cross-Linking
The two-step crosslinking method proceeds in two steps, the first step forming a weakly crosslinked, partially reacted polymer network, whose shape and orientation can be easily changed. Through mechanical stretching and other methods, monodomain LCEs with the desired shape can be obtained, and the shape and orientation are fixed by the second-step reaction. A typical example of two-step cross-linking was presented by Finkelmann et al., which was based on liquid crystal polysiloxanes [1][11]. In the presence of a platinum catalyst, linear polysiloxane chains are mixed and reacted with vinyl liquid crystal monomers and multifunctional vinyl crosslinkers (Figure 2a). Taking advantage of the difference in the reaction rates of the two different cross-linking agents, the first-step reaction produces weakly cross-linked polymers, and aligns the polymer chains by mechanical stretching. Under the condition of weight loading, the second part of the polymerization reaction forms a strong cross-linked polymer network and permanently maintains a uniform director orientation.
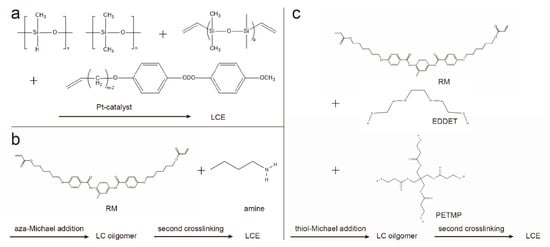
Figure 2. Synthetic route for two-step cross-linked LCE chemistry. (a) Two-step crosslinking by hydrosilylation; (b) aza-Michael addition between diacrylate-based RMs and amines; (c) thiol-Michael addition of diacrylate-based RMs and thiols.
The two-step hydrosilylation reaction route relies on non-commercially available materials. Therefore, the preparation of LCEs based on the chain extension reaction of diacrylate-based reactive mesogens (RMs) is gaining popularity, including the aza-Michael addition method [3][4][3,12] and thiol-Michael addition method [5][13]. Because both of the above mentioned methods are based on purchasable liquid crystal monomers and other components, the initiation conditions of the two-step polymerization are orthogonal based on the chain extension reaction, enabling easy access and good controllability. For the aza-Michael addition (Figure 2b), a classic example is based on the chain extension of liquid crystal diacrylate monomers via aza-Michael addition with amines [3][6][3,14], where the low viscosity of the reactive precursor and the slow reaction rate can be used for surface alignment [3][7][3,15]. The thiol-Michael addition reaction can be applied to chain extension to form oligomers (Figure 2c). After the alignment of oligomers, the orientation of the mesogens is then fixed by photopolymerization [8][9][16,17].
1.2. One-Step Cross-Linking
Low molar mass liquid crystals have been studied for many years and are frequently used in daily life due to optical anisotropy and electrical tunability. Alignment techniques for low molar mass liquid crystals are well developed [10][11][18,19], which can also be used to align low-viscosity precursors of LCEs. Compared with the two-step cross-linking method, the low molar mass liquid crystal monomers are directly polymerized to form LCE in the one-step cross-linking method without the process of chain growth and partial cross-linking, which results in better maintenance of orientation. It is worth noting that this alignment technique is usually based on a treated surface, which possesses strong programmability, but the anchoring force weakens with distance. Due to the limitation of the anchoring force, LCEs fabricated by this alignment method are thin, and the LCE precursor is required to have a low viscosity. In the one-step cross-linking method, the polymerizable liquid crystal monomer, cross-linking agent, and initiator are directly mixed. Since no pre-polymerization process is required and the viscosity of the precursor is low, alignment can be induced by a surface to achieve spatially complex orientation. The first example of a one-step cross-linked LCE was synthesized by free-radical polymerization of acrylates [12][2]. Thomsen et al. used acrylate-functionalized LC monomers for crosslinking to obtain side-chain LCEs. Orientation was based on surface-induced orientation, where the material was poured into glass cells coated with a rubbed polyvinyl alcohol film, then cooled from the isotropic phase (95 °C) to the nematic phase (85 °C) at −1 °C/min to obtain a good orientation. This method only takes a few minutes to obtain LCEs, which saves time compared to two-step crosslinking.
2. LCE-Based Composite Material
An indispensable part of photothermally driven LCEs materials are photothermal materials. Photothermal materials can absorb light of specific wavelengths, transform photon energy into heat energy, and transfer the heat to LCEs for driving. The introduction of some kinds of photothermal materials can enhance the mechanical properties of LCEs as well. The research on photothermal conversion materials ranges from inorganic to organic, including carbon-based materials, metal nanomaterials, and organic dyes.
