Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Pankaj Thakur and Version 4 by Vivi Li.
The demand for lightweight, high-modulus, and temperature-resistant materials for aerospace and other high-temperature applications has contributed to the development of ceramic fibers that exhibit most of the favorable properties of monolithic ceramics. The preceramic polymer requirements for a fiber concoction include: (1) appropriate rheology for non-Newtonian flows of materials and appropriate viscosity during rotation; (2) reactivity to fuse the fibers for subsequent pyrolysis; (3) controlled degradation during pyrolysis to prevent disorders of the structure, such as scattered material, and to produce high-density fibers with high ceramic performance; (4) controlled formation of nano- or microstructures.
- preceramic
- polymer fiber
- spinning polymer fibers
- electrospinning
- wet spinning
- ceramic fibers
- ceramic fibers applications
- high-temperature ceramics
1. Introduction
The demand for lightweight, high-modulus, and temperature-resistant materials for aerospace and other high-temperature applications has contributed to the development of ceramic fibers. The ceramic fiber materials demonstrate monolithic characteristics [1][2][3][4]; however, they are somewhat breakable. Developing these materials results in high-performance ceramic fibers that can be produced economically. Fillers, additives, and inorganic polymeric materials are essential to making ceramic fibers. An overview of preceramic fiber materials and fabrication approaches is presented in this section [1][5][6][7].
Ceramic fibers include all non-metallic inorganic fibers (oxide or non-oxide) except for fibers manufactured via solidifying glass melts. Organic, polymeric fiber materials cannot be used in ceramic matrix composites (CMCs), because their degradation temperatures are below 500 °C; neither can conventional glass fibers with melting or softening points below 700 °C be used [8][9][10][11][12][13][14].
A perfect fiber structure cannot be practically obtained during processing; thus, the fiber properties are usually well below the theoretical property values calculated for a perfect design. An important goal of fiber spinning and fiber formation is the minimization of structural imperfections through the optimization of production processes. Highly optimized and sophisticated processes are usually more important in high-performance fiber production than the materials used. Thus, manufacturing processes should be examined for flaws or inconsistencies that may compromise fiber quality.
Manufacturing processes for inorganic fibers can be categorized as indirect or direct. An indirect process involves coating fiber materials to produce fibers or non-ceramic precursor fibers. Inorganic fibers are formed by pyrolysis of an organic template fiber, which is then soaked in preceramic precursor materials, or precursor materials are deposited on its surface. A direct process involves the direct spinning of inorganic precursors (solutions of salt, suns, or molten precursors) into “green fibers”, sometimes using organic polymer additives [15][16][17][18][19][20].
The first step in producing non-oxide ceramic fibers is the synthesis of inorganic polymers which have compositions that are determined by the precursor polymers and lengths that depend on the production process. Precursor polymers of non-oxide ceramic materials, such as polysilanes, polycarbosilanes, polysilazanes, polycarbosilazanes, and polyborosilazanes, are organosilicon compounds [21][22][23][24][25][26]. The ceramic composition is determined by the type of precursor ceramic used. The polymer precursor structure should be carefully constructed to obtain ceramic fibers with the desired properties. The preceramic polymer requirements for a fiber concoction include: (1) appropriate rheology for non-Newtonian flows of materials and appropriate viscosity during rotation; (2) reactivity to fuse the fibers for subsequent pyrolysis; (3) controlled degradation during pyrolysis to prevent disorders of the structure, such as scattered material, and to produce high-density fibers with high ceramic performance; (4) controlled formation of nano- or microstructures. Silicon carbide ceramics and fibers are produced using polysilanes and polycarbosilanes as precursor polymers. Polycarbosilanes are sometimes modified by adding metal-organic compounds and other polymers such as polypropylene or polymethylphenylsiloxane. Supplementing polycarbosilanes with polymethylphenylsiloxane results in oxygen-rich SiC fibers.
SiCN-based ceramics and fibers are produced using polycarbosilazanes as precursor materials; Si3N4-based materials are made using polysilazanes as precursors.
Production processes can differ based on the fiber lengths produced. There are production processes for continuous and short fibers with ranges from millimeters to centimeters. Continuous fibers are generated through the conventional spinning approach, and short fibers are spun using fast-rotating discs or blowing air. The fibers produced from non-oxide fibers have better mechanical strength and modulus values than traditional oxide fibers because of their chemical structure, which can switch between polycrystalline or amorphous. Amorphous fibers have lower creep rates than polycrystalline oxide fibers at high temperatures. Thus, non-oxide fibers have application restrictions due to the oxidative degradation [27][28][29]. Fibers with a lower oxygen content have greater oxidation resistance. Silicon-based non-oxide ceramics, such as silicon carbide (SiC), silicon oxycarbide (SiOC), silicon nitride (Si3N4), and their derivatives (SiCN, SiAlON, etc.), are used in a wide range of applications for their heat resistance, chemical stability, excellent mechanical or electrical properties, and other characteristics [30][31][32][33][34][35][36]. Polymers containing organosilicon have been successfully synthesized and used to produce silicone-based ceramics for more than 55 years [37][38][39]. Production of silicon-based ceramics derived from organosilicon polymers generally includes cross-linking, pyrolysis, and ceramization [40][41][42].
Preceramic polymers with novel properties have been developed in recent decades, laying a foundation for ceramic fiber production. Polymer-derived ceramic (PDC) fiber production includes three steps: (i) synthesizing/modifying preceramic polymers; (ii) spinning (melt spinning or electrospinning) and solidifying polymer fibers; (iii) pyrolyzing polymer fibers into ceramic fibers. The combined efforts of chemists, materials scientists, and engineers have produced ceramic fibers with different properties [43][44][45][46][47][48][49][50]. Ceramic fibers can maintain their mechanical properties up to 2000 °C with excellent process oxidation, corrosion resistance, and nanosized structures. They can be used in traditional CMCs and new applications including energy conversion and storage devices. This entry presents an overview of PDC ceramic fiber development and future research. This entry discusses the functional chemistry, molecular weight influences, surface modification, and on-demand application of preceramic fibers. The preceramic polymer fiber fabrication methods and application of these fibers are presented in Figure 1.
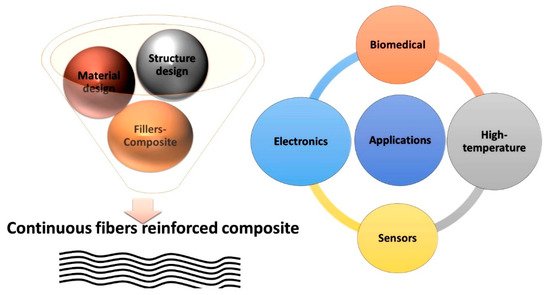
Figure 1. Preceramic polymer fiber fabrication methods and their applications.
2. Discussion
2.1. Production of Fibers
Fiber spinning can be generalized into two primary categories: solution processes, such as dry or wet spinning, and melt spinning. In solution processes, a polymer is dissolved in a solvent to form a “dope” and then extruded through an orifice where fiber consolidation/solidification is triggered through evaporation (i.e., dry process) or coagulation (i.e., wet process). Fiber solidification, in either case, depends on heat and mass transfer; these processes are complex and are used for materials that do not melt/flow easily or that thermally degrade at typical processing temperatures. Consequently, production tends to be slower and costlier. In melt spinning, fiber solidification occurs by cooling the fiber line after extrusion when the polymer is below its melting point; no solvent is present in the polymer melt. Melt-spinning processes are standard industrial processes for many thermoplastics, as they are often the fastest and simplest forming technique for fiber production, relying only on heat transport for solidification [51][52][53][54]. In polymer precursor methods for ceramics, the polymer is shaped using a fiber-spinning method and typically cross-linked before subsequent conversion to a ceramic. Numerous processing methods are available to produce these fibers including thaw spinning [33], sol-gel processes [41][55][56] electrospinning, and blow spinning. An overview of fiber-spinning methods is presented in Figure 2 [41][57].
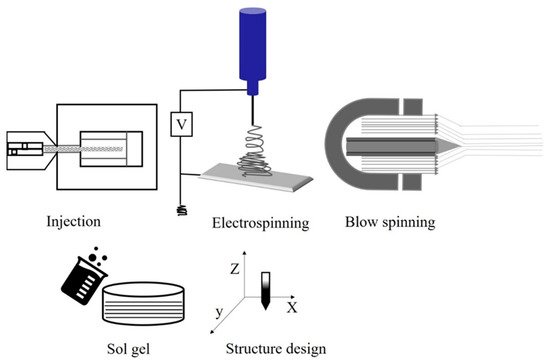
Figure 2. Methods to fabricate ceramic-based polymer fiber structures.
2.1.1. Electrospinning
Electrospinning is an adaptable and tunable method that uses a simple configuration and process to extract micron to submicron fibers from polymer solutions or molten polymers. All SiC, SiCN, and SiBCN ceramic fibers were once fabricated through melt spinning with a diameter of several microns. Recently, the fabrication of non-oxide ceramic fibers through electrospinning has become popular, as electrospinning can generate polymer fibers with good control of the fiber size and morphology. Similar to melt spinning, appropriate polymer structures are required to obtain uniform electrospun polymer fibers. Theoretical and experimental studies have indicated that the elasticity and entanglement of polymer chains are crucial in obtaining uniform fibers.
Electrospinning has been considered the most promising method for producing continuous fibers. It is also one of the most customizable continuous fiber production methods; the fiber diameter can be adjusted from micrometers to nanometers [58]. In addition, electrospinning can be used to synthesize composite and single-phase fibers. Electrospinning can produce fibers with different composite structure architectures, such as porous core–shell, hollow-shell, stripe-shaped, spongy, core–shell, and hollow structures. There is the possibility of generating nanofibrous layers with a designed aggregate structure including orientation, modeling, and two- and three-dimensional nanonets. In past years, more effort has been invested in producing ceramic fibers using the electrospinning technique [59]. Fibers spun by an electrospinning process are classified according to their structure (micro/nano) and properties, fibers morphology, and chemical structure. These fibers can be used in many applications. The superficial oxide ceramic fibers, such as TiO2, Al2O3, and ZnO, and complex oxide ceramic fibers, such as CaCu3Ti4O12 and Li1.6Al0.6MnO4, are produced by the electrospinning method [59]. Further, ZrC and Cu2ZnSnS4, which are non-oxide ceramic fibers, are made via the electrospinning process. Table 1 presents several recent simple and complex oxide and non-oxide ceramic fibers produced by electrospinning [60].
Table 1. Comparison of electrospinning and blow spinning techniques [61].
| Fabrication | Blow Spinning | Electrospinning |
|---|---|---|
| diameter | 80–1000 nm | 10–800 nm |
| mean pore size | 6–18 μm | 5 μm |
| porosity | ~97% | 70% |
Sneddon, Allcock et al. pioneered the production of boron carbide ceramic nanofibers using a single-source polymer precursor polymer from poly(norbornenlyldecaborane) (PND, molecular weight = 32 kDa) [6162]. The green boron carbide fibers are drawn through pyrolysis at 1000–1300 °C. SiC electrospinning fibers were drawn by Youngblood et al. using the spinning preceramic polymer of poly(carbomethylsilane) (PCmS). Polystyrene (PS) polymeric-based additives were added to the lower molecular weight of PCmS (3500 Da) polymer solutions to enhance spinnability. Salles et al. describe the submicron-diameter fiber production of novel boron nitride (BN) from a polymer blend solution of polyacrylonitrile (PAN) and poly((B-(methylamino)borazine) [6263]. Producing SiCN fibers through electrospinning a commercially available preceramic material, Ceraset™, is a cost-effective fabrication approach. A unique and simple technique has been developed to fabricate Si–C–N–Al ceramic nonwoven mats via electrospinning aluminum-functionalized oligosilazane [6364]. Blending preceramic polymers with other commonly used polymers followed by electrospinning can generate nanostructures in polymer fibers through the phase separation between different polymers. This approach allows for the fabrication of ceramic fibers with nanosized structures that can be used in applications where a large surface area is desired. Polyureasilazane (PUS), a low-viscosity thermoset PDC precursor, was blended with poly(methylmethacrylate) (PMMA) [6465]. Similarly, polymer-derived silicon oxycarbide (SiCO) ceramic-doped carbon fibers with an average diameter of 165 nm were successfully fabricated using simple electrospinning mixtures of PUS and PAN, a commonly available carbon fiber precursor polymer, followed by oxidative stabilization and pyrolysis at 1000 °C in air and Ar, respectively [6566]. Electrospinning of preceramic polymers offers a versatile and controllable approach for producing nanosized ceramic fibers that cannot be achieved by other methods. Integration of other functional materials into polymer fibers introduces additional functionality and structure with possibilities for new applications.
3. Influence of Polymer Structure
Branching has a profound effect on the processing of precursor polymers for ceramics. One of the challenges in converting these materials is shape retention during pyrolysis after the initial forming process. Fibers undergo volumetric shrinkage due to the loss of low-molecular-weight volatiles and deformation due to the fact of melting (if semi-crystalline) or creep at high temperatures. Branching significantly increases the molecular weight of the polymer and creates a more amorphous structure that does not melt at high temperatures [6667][6768]. With increased molecular weight and degree of entanglement due to the change in molecular structure, branched polymers have a higher viscosity that is more amenable to fiber-spinning processes. Laine et al. investigated the effect of linear and star-branched structures on solution-spun polymethylsilane; see the summarized chemical structure in Figure 3. The linear version of the polymer melted and did not have residual reactivity to prevent melting by any other means.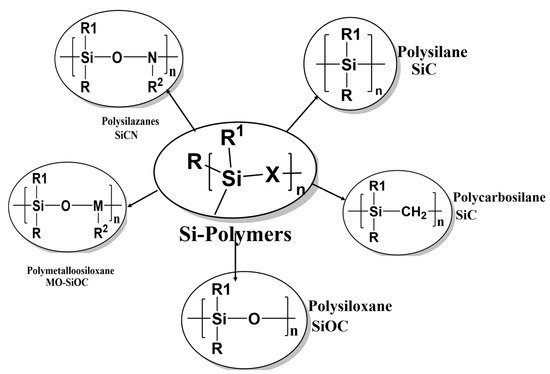
Figure 3. Ceramic conversion of Si-polymers with branched structures.
4. Polymer Backbone Structure
Substitution of polymer structure in BN precursors plays an essential role in creating a melt-spinnable polymer. In BN precursors, cross-linking occurs readily between N-H and B-H couples, forming hydrogen gas. The chemical structure of the backbone is illustrated in Figure 4. However, the cross-linking occurs at low temperatures, preventing melt spinning at higher temperatures. One approach is to reduce the number of reactive sites capable of cross-linking by substituting in the long-chain aliphatic pendant group [7071]. This method was reported to help plasticize the polymer melt and reduce cross-linking during processing such that melt-spun fibers could be formed. Unreacted pendant groups (–N(H)CH3) in the synthesis of poly(B-(methylamino)borazine) (Duperrier et al.) were thought to help plasticize the polymer (specifically, lowering the glass transition); the best fibers produced from these materials had an intermediate number of –N(H)CH3 pendant groups and flexible –N(CH3) bridges balanced with cross-links (B-N) between neighboring borazines [7172].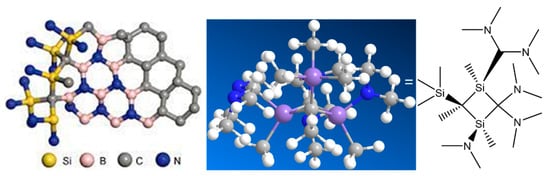
Figure 4. Structure of polymer backbone-derived SiBCN ceramics and functional siloxanes chemistry.
The most advantageous feature of polymer-derived multicomponent ceramic fibers is the incorporation of carbon or boron in silica and silicon nitride, which cannot be achieved through synthetic approaches or traditional solid-state reactions. The chemical formulation of silicon nitride and carbon in silica substantially influences ceramic fibers’ chemical structure and properties. Carbon hinders the crystallization of SiO2 and Si3N4 up to 1500 °C. Boron-containing SiCN exhibits more excellent thermal stability and crystallizes at approximately 1700 °C, granting multicomponent ceramic fibers an amorphous matrix for high-temperature applications free of grain-boundary-related properties such as corrosion, grain growth, and creep. In addition, multicomponent ceramic fibers demonstrate excellent tolerance to oxygen, which causes severe degradation of polycrystalline ceramics at high temperatures. The difference in the high-temperature behavior of Nicalon and Hi-Nicalon SiC fibers indicates that eliminating oxygen is crucial for improving creep resistance and crystallization resistance at high temperatures. Pendant groups have also been used to incorporate small amounts of borane into SiC, SiN, and their composites produced through preceramic processes. Wideman et al. added boron-containing pendant groups to a hydridopolysilazane, as boron is known to reduce crystallization in SiC and SiN. Although the addition of borazine resulted in too much residual reactivity for melt-processing, using a monofunctional borane was successful; the material exhibited a greater mass loss during heating but was melt-spinnable [7273].
Replacing reactive B-H or N-H groups with a long-chain aliphatic improved spinnability by reducing cross-linking sites (to allow higher temperature processing) and enhanced plasticity.
 Early on, it was recognized that adding suitable filler materials to a preceramic polymer could lead to bulk components that retain their integrity after pyrolysis [7475]. Two types of fillers can be used for this purpose: (1) “inert”, or passive fillers, which are ceramic powders that do not react with the ceramic residue from the preceramic polymer, the decomposition gases, or the heating atmosphere, and (2) “active” fillers, which are metallic or intermetallic powders that react during pyrolysis with decomposition gases generated during heating, the heating atmosphere, or (more rarely) with the ceramic residue from the preceramic polymer [75][76][77][78][79][80].
Mixing preceramic polymers and nanosized active filler particles enables the fabrication of polymer-derived silicate ceramics in different shapes and compositions that can be used in many engineering and specialized applications. The nanosized active filler particles mixed with preceramic polymers facilitate the fabrication of polymer-derived silicate ceramics in different shapes and compositions that can be used in many engineering and specialized applications. Key features of this approach include (a) a low heat required to generate the desired phases, stemming from the high reactivity of the preceramic polymer–ceramic residue with nanosized fillers; (b) suitable mixtures and processing conditions that favor the production of phase-pure components; (c) the possibility of shaping the mixtures into complex 3D bodies using a wide range of plastic-forming and other processing techniques.
Early on, it was recognized that adding suitable filler materials to a preceramic polymer could lead to bulk components that retain their integrity after pyrolysis [7475]. Two types of fillers can be used for this purpose: (1) “inert”, or passive fillers, which are ceramic powders that do not react with the ceramic residue from the preceramic polymer, the decomposition gases, or the heating atmosphere, and (2) “active” fillers, which are metallic or intermetallic powders that react during pyrolysis with decomposition gases generated during heating, the heating atmosphere, or (more rarely) with the ceramic residue from the preceramic polymer [75][76][77][78][79][80].
Mixing preceramic polymers and nanosized active filler particles enables the fabrication of polymer-derived silicate ceramics in different shapes and compositions that can be used in many engineering and specialized applications. The nanosized active filler particles mixed with preceramic polymers facilitate the fabrication of polymer-derived silicate ceramics in different shapes and compositions that can be used in many engineering and specialized applications. Key features of this approach include (a) a low heat required to generate the desired phases, stemming from the high reactivity of the preceramic polymer–ceramic residue with nanosized fillers; (b) suitable mixtures and processing conditions that favor the production of phase-pure components; (c) the possibility of shaping the mixtures into complex 3D bodies using a wide range of plastic-forming and other processing techniques.
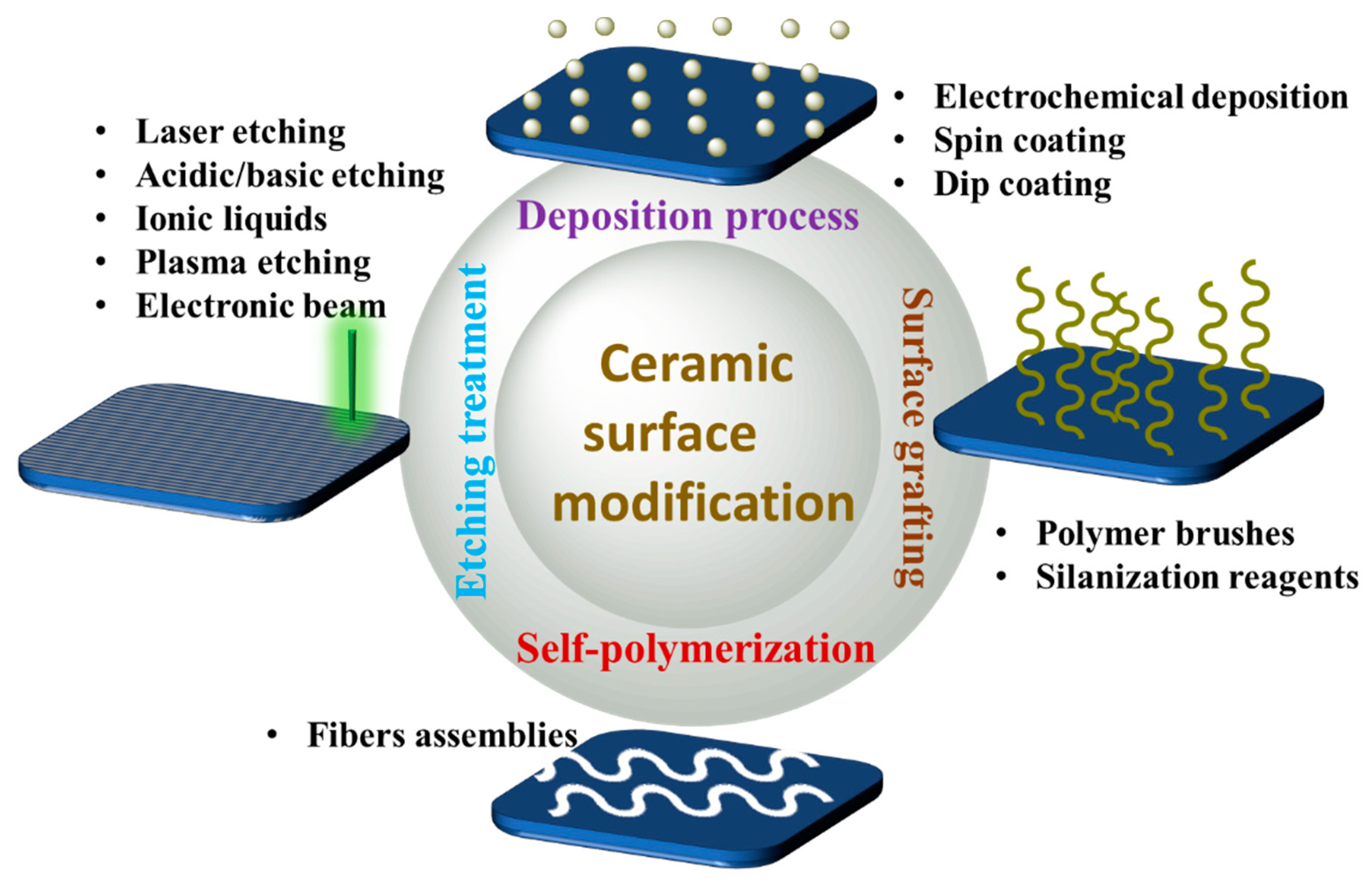 Chemical etching: Chemical etching is an old method of surface modification method. Several factors are included in this process such as chemical composition, heat, and concentration of reactants in the etching/electrochemical baths. Different reagents can be used for this process including acids and bases [80][81][82].
Silanization: Silanization is a process commonly used in current research due to the fact of its many advantages. Surface modification via the silanization method can be performed on powder or solid material. The widely used reagent for the silanization process is organic silane, which can create a monolayer assembly on the surfaces [83][84][85][86][87][88][89][90]. The surface-modified material can enhance and stimulate adhesion to surface/materials. Several commercially available compounds with modified silane surfaces include the functional groups thiol, amine, epoxide, and carboxyl.
Laser: Laser surface modification is a unique way of modifying the material’s chemistry, and this process does not make contact with the materials, unlike the silanization and etching process. A process laser can be used as external stimuli to change the surface chemistry remotely without touching and disturbing the material surface [91]. In addition, laser treatment plays a crucial function in supporting surface chemistry. Altering the surface properties can improve the surface wettability of the materials.
Coating/grafting: Plasma treatment is the most common process used in industries for the coating/grafting method. Grafting/coating provides bond strength and better mechanical properties, giving good deposition at a low cost. One of the advantages of this coating process is that thickness can be adjustable according to the requirements [92][93].
Electrochemical deposition: The electrophoretic deposition process was introduced to simplify the coating method, an alternative to the traditional coating method that is more straightforward and versatile than the conventional coating [94][95]. This method is broadly used to obtain monolayer coatings on ceramic fibers or substrates.
Self-assembly: Self-assembly is an autonomous process that organizes a structure or pattern without secondary interference [96]. The monolayer assembly forms naturally upon deposition by spraying active organic compounds onto solid surfaces [97][98]. The main (head) group of the surfactant and the character of the substrate is the main driving force in creating self-assembly. There are three parts in surfactant that help to form an assembly structure: (1) the leading surface group (active head) that attaches to the surface structure; (2) the interfacial properties of the assembly, which are side (terminal) groups; (3) the alkaline chains that link the main and side (head and the terminal) groups. The Van der Waals interface can impact the alkyl chains that orient and stabilize the monolayers. Many factors must be considered when creating a good self-assembly monolayer such as functional chemistry, adhesion and interaction with molecules, solvents, and substrate surface mobility [99][100][101][102].
Chemical etching: Chemical etching is an old method of surface modification method. Several factors are included in this process such as chemical composition, heat, and concentration of reactants in the etching/electrochemical baths. Different reagents can be used for this process including acids and bases [80][81][82].
Silanization: Silanization is a process commonly used in current research due to the fact of its many advantages. Surface modification via the silanization method can be performed on powder or solid material. The widely used reagent for the silanization process is organic silane, which can create a monolayer assembly on the surfaces [83][84][85][86][87][88][89][90]. The surface-modified material can enhance and stimulate adhesion to surface/materials. Several commercially available compounds with modified silane surfaces include the functional groups thiol, amine, epoxide, and carboxyl.
Laser: Laser surface modification is a unique way of modifying the material’s chemistry, and this process does not make contact with the materials, unlike the silanization and etching process. A process laser can be used as external stimuli to change the surface chemistry remotely without touching and disturbing the material surface [91]. In addition, laser treatment plays a crucial function in supporting surface chemistry. Altering the surface properties can improve the surface wettability of the materials.
Coating/grafting: Plasma treatment is the most common process used in industries for the coating/grafting method. Grafting/coating provides bond strength and better mechanical properties, giving good deposition at a low cost. One of the advantages of this coating process is that thickness can be adjustable according to the requirements [92][93].
Electrochemical deposition: The electrophoretic deposition process was introduced to simplify the coating method, an alternative to the traditional coating method that is more straightforward and versatile than the conventional coating [94][95]. This method is broadly used to obtain monolayer coatings on ceramic fibers or substrates.
Self-assembly: Self-assembly is an autonomous process that organizes a structure or pattern without secondary interference [96]. The monolayer assembly forms naturally upon deposition by spraying active organic compounds onto solid surfaces [97][98]. The main (head) group of the surfactant and the character of the substrate is the main driving force in creating self-assembly. There are three parts in surfactant that help to form an assembly structure: (1) the leading surface group (active head) that attaches to the surface structure; (2) the interfacial properties of the assembly, which are side (terminal) groups; (3) the alkaline chains that link the main and side (head and the terminal) groups. The Van der Waals interface can impact the alkyl chains that orient and stabilize the monolayers. Many factors must be considered when creating a good self-assembly monolayer such as functional chemistry, adhesion and interaction with molecules, solvents, and substrate surface mobility [99][100][101][102].
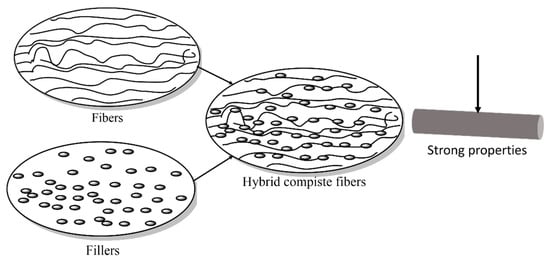
5. Interaction with Fillers and Fibers
Composites with fillers or additives often provide better performance than pure polymers. Fillers are classified according to size as macro-, micro-, and nanofillers. Nano-filler-reinforced material offers significantly better performance than micro-and macro-fillers at the same filler loading [7374]. How fillers can increase the properties of the materials is illustrated in Figure 5.
Figure 5. Effect of micro- and nanofillers on the mechanical properties of hybrid nanocomposites.
6. Surface Modification
Modifying the surfaces of ceramic-based materials can modify the properties of the materials. The surface-modified material will enable changes in flexibility and offer hydrophilic (amines, carboxylic acids) and hydrophobic (hydrocarbons or silane-based materials) characteristics. Several methods have been used to modify the surfaces, such as oxidation, etching with ionic liquids, silanization, plasma treatment, and thermal and laser process; see Figure 6. Here, researchers discuss the two most frequently used surface modification methods.
Figure 6. The different methods used for surface modification.
References
- Wang, J.-C.; Dommati, H.; Hsieh, S.-J. Review of additive manufacturing methods for high-performance ceramic materials. Int. J. Adv. Manuf. Technol. 2019, 103, 2627–2647.
- Nishihora, R.K.; Rachadel, P.L.; Quadri, M.G.N.; Hotza, D. Manufacturing porous ceramic materials by tape casting—A review. J. Eur. Ceram. Soc. 2018, 38, 988–1001.
- Bajraktarova-Valjakova, E.; Korunoska-Stevkovska, V.; Kapusevska, B.; Gigovski, N.; Bajraktarova-Misevska, C.; Grozdanov, A. Contemporary Dental Ceramic Materials, A Review: Chemical Composition, Physical and Mechanical Properties, Indications for Use. Open Access Maced. J. Med Sci. 2018, 6, 1742–1755.
- Rakshit, R.; Das, A.K. A review on cutting of industrial ceramic materials. Precis. Eng. 2019, 59, 90–109.
- Lakhdar, Y.; Tuck, C.; Binner, J.; Terry, A.; Goodridge, R. Additive manufacturing of advanced ceramic materials. Prog. Mater. Sci. 2020, 116, 100736.
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549.
- Cramer, C.L.; Ionescu, E.; Graczyk-Zajac, M.; Nelson, A.T.; Katoh, Y.; Haslam, J.J.; Wondraczek, L.; Aguirre, T.G.; LeBlanc, S.; Wang, H.; et al. Additive manufacturing of ceramic materials for energy applications: Road map and opportunities. J. Eur. Ceram. Soc. 2022, 42, 3049–3088.
- Clauß, B. Fibers for Ceramic Matrix Composites; Wiley-VCH: Weinheim, Germany, 2008.
- Liu, Y.; Jiang, X.; Shi, J.; Luo, Y.; Tang, Y.; Wu, Q.; Luo, Z. Research on the interface properties and strengthening–toughening mechanism of nanocarbon-toughened ceramic matrix composites. Nanotechnol. Rev. 2020, 9, 190–208.
- Du, J.; Zhang, H.; Geng, Y.; Ming, W.; He, W.; Ma, J.; Cao, Y.; Li, X.; Liu, K. A review on machining of carbon fiber reinforced ceramic matrix composites. Ceram. Int. 2019, 45, 18155–18166.
- Diaz, O.G.; Luna, G.G.; Liao, Z.; Axinte, D. The new challenges of machining Ceramic Matrix Composites (CMCs): Review of surface integrity. Int. J. Mach. Tools Manuf. 2019, 139, 24–36.
- Arai, Y.; Inoue, R.; Goto, K.; Kogo, Y. Carbon fiber reinforced ultra-high temperature ceramic matrix composites: A review. Ceram. Int. 2019, 45, 14481–14489.
- Binner, J.; Porter, M.; Baker, B.; Zou, J.; Venkatachalam, V.; Diaz, V.R.; D’Angio, A.; Ramanujam, P.; Zhang, T.; Murthy, T.S.R.C. Selection, processing, properties and applications of ultra-high temperature ceramic matrix composites, UHTCMCs—A review. Int. Mater. Rev. 2019, 65, 389–444.
- An, Q.; Chen, J.; Ming, W.; Chen, M. Machining of SiC ceramic matrix composites: A review. Chin. J. Aeronaut. 2020, 34, 540–567.
- Devasia, R.; Painuly, A.; Devapal, D.; Sreejith, K. Continuous fiber reinforced ceramic matrix composites. In Fiber Reinforced Composites; Woodhead Publishing: Sawston, UK, 2021; pp. 669–751.
- Ferreira, R.K.M.; Ramlow, H.; Marangoni, C.; Machado, R.A.F. A review on the manufacturing techniques of porous hydrophobic ceramic membranes applied to direct contact membrane distillation. Adv. Appl. Ceram. 2021, 120, 336–357.
- Henning, L.M.; Abdullayev, A.; Vakifahmetoglu, C.; Simon, U.; Bensalah, H.; Gurlo, A.; Bekheet, M.F. Review on Polymeric, Inorganic, and Composite Materials for Air Filters: From Processing to Properties. Adv. Energy Sustain. Res. 2021, 2, 2100005.
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: “All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856.
- Chawla, K.K. Composite Materials: Science and Engineering; Springer: New York, NY, USA, 2012; Available online: https://books.google.com/books?hl=en&lr=&id=rbuNxwzM27cC&oi=fnd&pg=PR7&dq=These+fibers+are+made+by+soaking+with+preceramic+precursor+materials+or+depositing+precursor+materials+on+the+surface.+The+inorganic+fiber+is+then+formed+by+pyrolysis+of+the+organic+template+fiber.+On+the+other+hand,+the+direct+process+involves+the+direct+&ots=UphMpqJeh-&sig=SA43ZjxxkfMVB2H8__8vOfmj0O8#v=onepage&q&f=false (accessed on 8 May 2022).
- Fang, W.; Yang, S.; Wang, X.L.; Yuan, T.Q.; Sun, R.C. Manufacture and application of lignin-based carbon fibers (LCFs) and lignin-based carbon nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827.
- Whitaker, T.W.; Cutler, H.C. Cucurbits from Preceramic Levels at Guilá Naquitz. In Guilá Naquitz; Routledge: London, UK, 2021; pp. 275–279.
- Chen, H.; Wang, X.; Xue, F.; Huang, Y.; Zhou, K.; Zhang, D. 3D printing of SiC ceramic: Direct ink writing with a solution of preceramic polymers. J. Eur. Ceram. Soc. 2018, 38, 5294–5300.
- Cagnato, C. Gathering and Sowing Across the Central Maya Lowlands: A Review of Plant Use by Preceramic Peoples and The Early to Middle Preclassic Maya. Anc. Mesoam. 2021, 32, 486–501.
- Hotza, D.; Nishihora, R.K.; Machado, R.; Geffroy, P.; Chartier, T.; Bernard, S. Tape casting of preceramic polymers toward advanced ceramics: A review. Int. J. Ceram. Eng. Sci. 2019, 1, 21–41.
- Smith, C.E. Preceramic Plant Remains from Guilá Naquitz. In Guilá Naquitz; Routledge: London, UK, 2021; pp. 265–274.
- Bernardo, E.; Fiocco, L.; Parcianello, G.; Storti, E.; Colombo, P. Advanced Ceramics from Preceramic Polymers Modified at the Nano-Scale: A Review. Materials 2014, 7, 1927–1956.
- Sujith, R.; Jothi, S.; Zimmermann, A.; Aldinger, F.; Kumar, R. Mechanical behaviour of polymer derived ceramics—A review. Int. Mater. Rev. 2020, 66, 426–449.
- Scholz, H.; Vetter, J.; Herborn, R.; Ruedinger, A. Oxide ceramic fibers via dry spinning process—From lab to fab. Int. J. Appl. Ceram. Technol. 2020, 17, 1636–1645.
- Viard, A.; Fonblanc, D.; Lopez-Ferber, D.; Schmidt, M.; Lale, A.; Durif, C.; Balestrat, M.; Rossignol, F.; Weinmann, M.; Riedel, R.; et al. Polymer Derived Si-B-C-N Ceramics: 30 Years of Research. Adv. Eng. Mater. 2018, 20, 1800360.
- Zhao, Z.; Li, K.; Li, W.; Liu, Q.; Kou, G.; Zhang, Y. Preparation, ablation behavior and mechanism of C/C-ZrC-SiC and C/C-SiC composites. Ceram. Int. 2018, 44, 7481–7490.
- Du, B.; Hong, C.; Zhang, X.; Wang, J.; Qu, Q. Preparation and mechanical behaviors of SiOC-modified carbon-bonded carbon fiber composite with in-situ growth of three-dimensional SiC nanowires. J. Eur. Ceram. Soc. 2018, 38, 2272–2278.
- Zhang, L.; Pei, L.; Li, H.; Li, S.; Liu, S.; Guo, Y. Preparation and characterization of Na and F co-doped hydroxyapatite coating reinforced by carbon nanotubes and SiC nanoparticles. Mater. Lett. 2018, 218, 161–164.
- Wen, Q.; Yu, Z.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2020, 109, 100623.
- Chen, A.-N.; Wu, J.-M.; Han, L.-X.; Cheng, L.-J.; Liu, R.-Z.; Zhang, X.-Y.; Shi, Y.-S.; Li, C.-H. Preparation of Si3N4 foams by DCC method via dispersant reaction combined with protein-gelling. J. Alloys Compd. 2018, 745, 262–270.
- Han, L.; Wang, J.; Li, F.; Wang, H.; Deng, X.; Zhang, H.; Zhang, S. Low-temperature preparation of Si3N4 whiskers bonded/reinforced SiC porous ceramics via foam-gelcasting combined with catalytic nitridation. J. Eur. Ceram. Soc. 2018, 38, 1210–1218.
- Nguyen, V.L.; Zera, E.; Perolo, A.; Campostrini, R.; Li, W.; Sorarù, G.D. Synthesis and characterization of polymer-derived SiCN aerogel. J. Eur. Ceram. Soc. 2015, 35, 3295–3302.
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908.
- Greil, P. Polymer Derived Engineering Ceramics. Adv. Eng. Mater. 2000, 2, 339–348.
- Bansal, N.P.; Boccaccini, A.R. (Eds.) Ceramics and Composites Processing Methods; Wiley: Hoboken, NJ, USA, 2012; Available online: https://www.wiley.com/en-us/Ceramics+and+Composites+Processing+Methods-p-9780470553442 (accessed on 6 June 2021).
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A,. 2019, 7, 1936–1963.
- Janakiraman, N.; Aldinger, F. Fabrication and characterization of fully dense Si–C–N ceramics from a poly (ureamethylvinyl) silazane precursor. J. Eur. Ceram. Soc. 2009, 29, 163–173.
- Yu, Z.; Yang, L.; Zhan, J.; Zhou, C.; Min, H.; Zheng, Q.; Xia, H. Preparation, cross-linking and ceramization of AHPCS/Cp2ZrCl2 hybrid precursors for SiC/ZrC/C composites. J. Eur. Ceram. Soc. 2012, 32, 1291–1298.
- Bechthold, M.; Weaver, J.C. Materials science and architecture. Nat. Rev. Mater. 2017, 2, 17082.
- Sigmund, W.; Yuh, J.; Park, H.; Maneeratana, V.; Pyrgiotakis, G.; Daga, A.; Taylor, J.; Nino, J.C. Processing and structure relationships in electrospinning of ceramic fiber systems. J. Am. Ceram. Soc. 2006, 89, 395–407.
- Zaarour, B.; Zhu, L.; Huang, C.; Jin, X.; Alghafari, H.; Fang, J.; Lin, T. A review on piezoelectric fibers and nanowires for energy harvesting. J. Ind. Text. 2019, 51, 297–340.
- Baldus, P.; Jansen, M.; Sporn, D. Ceramic Fibers for Matrix Composites in High-Temperature Engine Applications. Science 1999, 285, 699–703.
- Jia, C.; Xu, Z.; Luo, D.; Xiang, H.; Zhu, M. Flexible ceramic fibers: Recent development in preparation and application. Adv. Fiber Mater. 2022, 1–31.
- Gottardo, L.; Bernard, S.; Gervais, C.; Inzenhofer, K.; Motz, G.; Weinmann, M.; Balan, C.; Miele, P. Chemistry, structure and processability of boron-modified polysilazanes as tailored precursors of ceramic fibers. J. Mater. Chem. 2012, 22, 7739–7750.
- Swalen, J.D.; Allara, D.L.; Andrade, J.D.; Chandross, E.A.; Garoff, S.; Israelachvili, J.; McCarthy, T.J.; Murray, R.; Pease, R. Molecular monolayers and films. A panel report for the Materials Sciences Division of the Department of Energy. Langmuir 1987, 3, 932–950.
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298.
- Warlin, N.; Nilsson, E.; Guo, Z.; Mankar, S.V.; Valsange, N.G.; Rehnberg, N.; Lundmark, S.; Jannasch, P.; Zhang, B. Synthesis and melt-spinning of partly bio-based thermoplastic poly(cycloacetal-urethane)s toward sustainable textiles. Polym. Chem. 2021, 12, 4942–4953.
- Regnier, J.; Cayla, A.; Campagne, C.; Devaux, É. Melt Spinning of Flexible and Conductive Immiscible Thermoplastic/Elastomer Monofilament for Water Detection. Nanomaterials 2021, 12, 92.
- Tavanaie, M.A. Engineered biodegradable melt-spun fibers. In Engineered Polymeric Fibrous Materials; Woodhead Publishing: Sawston, UK, 2021; pp. 191–232.
- Ren, Z.; Gervais, C.; Singh, G. Fabrication and characterization of silicon oxycarbide fibre-mats via electrospinning for high temperature applications. RSC Adv. 2020, 10, 38446–38455.
- Lusha, Z.; Zicheng, T.; Tusiime, R.; Shaofei, W.; Ningning, F.; Yifan, Z.; Hui, Z.; Yong, L. Synthesis and electromagnetic wave absorbing properties of a polymer-derived SiBNC ceramic aerogel. Ceram. Int. 2021, 47, 18984–18990.
- Stojanovska, E.; Canbay, E.; Pampal, E.S.; Calisir, M.D.; Ağma, O.; Polat, Y.; Simsek, R.; Gundogdu, N.A.S.; Akgul, Y.; Kilic, A. A review on non-electro nanofibre spinning techniques. RSC Adv. 2016, 6, 83783–83801.
- Guo, A.; Roso, M.; Modesti, M.; Liu, J.; Colombo, P. Preceramic polymer-derived SiOC fibers by electrospinning. J. Appl. Polym. Sci. 2013, 131, 39836.
- Baji, A.; Mai, Y.-W. Engineering Ceramic Fiber Nanostructures Through Polymer-Mediated Electrospinning. In Polymer-Engineered Nanostructures for Advanced Energy Applications; Springer: Cham, Switzerland, 2017; pp. 3–30.
- Esfahani, H.; Jose, R.; Ramakrishna, S. Electrospun Ceramic Nanofiber Mats Today: Synthesis, Properties, and Applications. Materials 2017, 10, 1238.
- Welna, D.T.; Bender, J.D.; Wei, X.; Sneddon, L.G.; Allcock, H.R. Preparation of Boron-Carbide/Carbon Nanofibers from a Poly(norbornenyldecaborane) Single-Source Precursor via Electrostatic Spinning. Adv. Mater. 2005, 17, 859–862.
- Guron, M.M.; Wei, X.; Welna, D.; Krogman, N.; Kim, M.J.; Allcock, H.; Sneddon, L.G. Preceramic polymer blends as precursors for boron-carbide/silicon-carbide composite ceramics and ceramic fibers. Chem. Mater. 2009, 21, 1708–1715. Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber Assembly by Rotary Jet-Spinning. Nano Lett. 2010, 10, 2257–2261.
- Salles, V.; Bernard, S.; Brioude, A.; Cornu, D.; Miele, P. A new class of boron nitride fibers with tunable properties by combining an electrospinning process and the polymer-derived ceramics route. Nanoscale 2009, 2, 215–217. Guron, M.M.; Wei, X.; Welna, D.; Krogman, N.; Kim, M.J.; Allcock, H.; Sneddon, L.G. Preceramic polymer blends as precursors for boron-carbide/silicon-carbide composite ceramics and ceramic fibers. Chem. Mater. 2009, 21, 1708–1715.
- Lu, P.; Huang, Q.; Liu, B.; Bando, Y.; Hsieh, Y.-L.; Mukherjee, A.K. Macroporous Silicon Oxycarbide Fibers with Luffa-Like Superhydrophobic Shells. J. Am. Chem. Soc. 2009, 131, 10346–10347. Salles, V.; Bernard, S.; Brioude, A.; Cornu, D.; Miele, P. A new class of boron nitride fibers with tunable properties by combining an electrospinning process and the polymer-derived ceramics route. Nanoscale 2009, 2, 215–217.
- Lu, P.; Huang, Q.; Mukherjee, A.; Hsieh, Y.-L. SiCO-doped Carbon Fibers with Unique Dual Superhydrophilicity/Superoleophilicity and Ductile and Capacitance Properties. ACS Appl. Mater. Interfaces 2010, 2, 3738–3744. Lu, P.; Huang, Q.; Liu, B.; Bando, Y.; Hsieh, Y.-L.; Mukherjee, A.K. Macroporous Silicon Oxycarbide Fibers with Luffa-Like Superhydrophobic Shells. J. Am. Chem. Soc. 2009, 131, 10346–10347.
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. Lu, P.; Huang, Q.; Mukherjee, A.; Hsieh, Y.-L. SiCO-doped Carbon Fibers with Unique Dual Superhydrophilicity/Superoleophilicity and Ductile and Capacitance Properties. ACS Appl. Mater. Interfaces 2010, 2, 3738–3744.
- Sadeghi, F.; Ajji, A.; Carreau, P.J. Analysis of row nucleated lamellar morphology of polypropylene obtained from the cast film process: Effect of melt rheology and process conditions. Polym. Eng. Sci. 2007, 47, 1170–1178. Daristotle, J.L.; Behrens, A.M.; Sandler, A.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963.
- Mueller, C.; Capaccio, G.; Hiltner, A.; Baer, E. Heat sealing of LLDPE: Relationships to melting and interdiffusion. J. Appl. Polym. Sci. 1998, 70, 2021–2030. Sadeghi, F.; Ajji, A.; Carreau, P.J. Analysis of row nucleated lamellar morphology of polypropylene obtained from the cast film process: Effect of melt rheology and process conditions. Polym. Eng. Sci. 2007, 47, 1170–1178.
- Laine, R.M.; Zhang, Z.-F.; Rahn, J.A.; Chew, K.W.; Kannisto, M.; Scotto, C. Synthesis and Processing of SiC Based Materials Using Polymethylsilane. In Precursor-Derived Ceramics: Synthesis, Structures and High Temperature Mechanical Properties; Wiley-VCH: Weinheim, Germany, 1999; pp. 60–72. Mueller, C.; Capaccio, G.; Hiltner, A.; Baer, E. Heat sealing of LLDPE: Relationships to melting and interdiffusion. J. Appl. Polym. Sci. 1998, 70, 2021–2030.
- Suttor, D.; Hacker, J.; Traßl, S.; Müller, H.; Kleebe, H.-J.; Ziegler, G. Polymer Architecture and Crystallization Behavior of SiCN-Fiber Precursors. Ceram. Eng. Sci. Proc. 1997, 3, 127–133. Laine, R.M.; Zhang, Z.-F.; Rahn, J.A.; Chew, K.W.; Kannisto, M.; Scotto, C. Synthesis and Processing of SiC Based Materials Using Polymethylsilane. In Precursor-Derived Ceramics: Synthesis, Structures and High Temperature Mechanical Properties; Wiley-VCH: Weinheim, Germany, 1999; pp. 60–72.
- Mutlu, H.; de Espinosa, L.M.; Meier, M.A. Acyclic diene metathesis: A versatile tool for the construction of defined polymer architectures. Chem. Soc. Rev. 2011, 40, 1404–1445. Suttor, D.; Hacker, J.; Traßl, S.; Müller, H.; Kleebe, H.-J.; Ziegler, G. Polymer Architecture and Crystallization Behavior of SiCN-Fiber Precursors. Ceram. Eng. Sci. Proc. 1997, 3, 127–133.
- Duperrier, S.; Gervais, C.; Bernard, S.; Cornu, D.; Babonneau, F.; Balan, C.; Miele, P. Design of a Series of Preceramic B-Tri(Methylamine)Borazine-Based Polymers as Fiber Precursors: Achitecture, Thermal Behavior, and Melt-Spinnability. Macromolecules 2007, 40, 1018–1027. Mutlu, H.; de Espinosa, L.M.; Meier, M.A. Acyclic diene metathesis: A versatile tool for the construction of defined polymer architectures. Chem. Soc. Rev. 2011, 40, 1404–1445.
- Wideman, T.; Remsen, E.E.; Zank, G.A.; Sneddon, L.G. Polymeric Precursors for Bn and SiNCB Ceramic Fibers. In Precursor-Derived Ceramics: Synthesis, Structures and High Temperature Mechanical Properties; Wiley-VCH: Weinheim, Germany, 1999; pp. 103–112. Duperrier, S.; Gervais, C.; Bernard, S.; Cornu, D.; Babonneau, F.; Balan, C.; Miele, P. Design of a Series of Preceramic B-Tri(Methylamine)Borazine-Based Polymers as Fiber Precursors: Achitecture, Thermal Behavior, and Melt-Spinnability. Macromolecules 2007, 40, 1018–1027.
- Viard, A.; Miele, P.; Bernard, S. Polymer-derived ceramics route toward SiCN and SiBCN fibers: From chemistry of polycarbosilazanes to the design and characterization of ceramic fibers. J. Ceram. Soc. Jpn. 2016, 124, 967–980. Wideman, T.; Remsen, E.E.; Zank, G.A.; Sneddon, L.G. Polymeric Precursors for Bn and SiNCB Ceramic Fibers. In Precursor-Derived Ceramics: Synthesis, Structures and High Temperature Mechanical Properties; Wiley-VCH: Weinheim, Germany, 1999; pp. 103–112.
- Petrovic, J.J.; Vasudevan, A.K. Key developments in high temperature structural silicides. Mater. Sci. Eng. A 1999, 261, 1–5. Viard, A.; Miele, P.; Bernard, S. Polymer-derived ceramics route toward SiCN and SiBCN fibers: From chemistry of polycarbosilazanes to the design and characterization of ceramic fibers. J. Ceram. Soc. Jpn. 2016, 124, 967–980.
- Mirkhalaf, M.; Sarvestani, H.Y.; Yang, Q.; Jakubinek, M.B.; Ashrafi, B. A comparative study of nano-fillers to improve toughness and modulus of polymer-derived ceramics. Sci. Rep. 2021, 11, 6951. Petrovic, J.J.; Vasudevan, A.K. Key developments in high temperature structural silicides. Mater. Sci. Eng. A 1999, 261, 1–5.
- Sarraf, F.; Abbatinali, E.; Gorjan, L.; Sebastian, T.; Colombo, P.; Churakov, S.V.; Clemens, F. Effect of MgO sintering additive on mullite structures manufactured by fused deposition modeling (FDM) technology. J. Eur. Ceram. Soc. 2021, 41, 6677–6686. Mirkhalaf, M.; Sarvestani, H.Y.; Yang, Q.; Jakubinek, M.B.; Ashrafi, B. A comparative study of nano-fillers to improve toughness and modulus of polymer-derived ceramics. Sci. Rep. 2021, 11, 6951.
- Wei, Y.; Shi, Y.; Zhang, X.; Jiang, Z.; Zhang, Y.; Zhang, L.; Zhang, J.; Gong, C. Electrospinning of lightweight TiN fibers with superior microwave absorption. J. Mater. Sci. Mater. Electron. 2019, 30, 14519–14527. Sarraf, F.; Abbatinali, E.; Gorjan, L.; Sebastian, T.; Colombo, P.; Churakov, S.V.; Clemens, F. Effect of MgO sintering additive on mullite structures manufactured by fused deposition modeling (FDM) technology. J. Eur. Ceram. Soc. 2021, 41, 6677–6686.
- Gonzalo-Juan, I.; Riedel, R. Ceramic synthesis from condensed phases. ChemTexts 2016, 2, 1–21. Wei, Y.; Shi, Y.; Zhang, X.; Jiang, Z.; Zhang, Y.; Zhang, L.; Zhang, J.; Gong, C. Electrospinning of lightweight TiN fibers with superior microwave absorption. J. Mater. Sci. Mater. Electron. 2019, 30, 14519–14527.
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. Gonzalo-Juan, I.; Riedel, R. Ceramic synthesis from condensed phases. ChemTexts 2016, 2, 1–21.
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Wilwerding, T.M.; Latta, M.A.; Miyazaki, M. Interfacial Characteristics and Bond Durability of Universal Adhesive to Various Substrates. Oper. Dent. 2017, 42, E59–E70. Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837.
- Sattabanasuk, V.; Charnchairerk, P.; Punsukumtana, L.; Burrow, M.F. Effects of mechanical and chemical surface treatments on the resin-glass ceramic adhesion properties. J. Investig. Clin. Dent. 2017, 8, e12220.
- Sharma, J.R.; Bose, S.; Mandal, S.; Das, G.; Mukhopadhyay, S.; Barua, A. Influence of Acid and Alkali Etching on Sputtered Aluminium Doped Zinc Oxide Films. Mater. Today Proc. 2018, 5, 9726–9732.
- Vistas, C.R.; Águas, A.C.P.; Ferreira, G.N.M. Silanization of Glass Chips—A Factorial Approach for Optimization. Appl. Surf. Sci. 2013, 286, 314–318.
- Sánchez-Bodón, J.; del Olmo, J.A.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2021, 14, 165.
- Usman, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O.; Gbadamosi, A.O.; El Badawy, T.H.; Said, K.A.M. An overview of superhydrophobic ceramic membrane surface modification for oil-water separation. J. Mater. Res. Technol. 2021, 12, 643–667.
- Kollarigowda, R.H.; De Santo, I.; Rianna, C.; Fedele, C.; Manikas, A.C.; Cavalli, S.; Netti, P.A. Shedding light on azopolymer brush dynamics by fluorescence correlation spectroscopy. Soft Matter 2016, 12, 7102–7111.
- Kollarigowda, R.H.; Abraham, S.; Montemagno, C.D. Antifouling Cellulose Hybrid Biomembrane for Effective Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 29812–29819.
- Kollarigowda, R.H.; Bhyrappa, H.M.; Cheng, G. Stimulus-Responsive Biopolymeric Surface: Molecular Switches for Oil/Water Separation. ACS Appl. Bio Mater. 2019, 2, 4249–4257.
- Haney, R.; Kollarigowda, R.H.; Wiegart, L.; Ramakrishnan, S. Surface-Functionalized Cellulose Nanocrystals as Nanofillers for Crosslinking Processes: Implications for Thermosetting Resins. ACS Appl. Nano Mater. 2022, 5, 1891–1901.
- Kollarigowda, R.H.; Fedele, C.; Rianna, C.; Calabuig, A.; Manikas, A.C.; Pagliarulo, V.; Ferraro, P.; Cavalli, S.; Netti, P.A. Light-responsive polymer brushes: Active topographic cues for cell culture applications. Polym. Chem. 2017, 8, 3271–3278.
- Yasuno, K.; Kakura, K.; Taniguchi, Y.; Yamaguchi, Y.; Kido, H. Zirconia Implants with Laser Surface Treatment: Peri-Implant Bone Response and Enhancement of Osseointegration. J. Hard Tissue Biol. 2014, 23, 93–100.
- Lee, H.J.; Kim, S.-J.; Kim, Y.; Park, H.; Park, Y.-I.; Nam, S.E. Effect of coating and surface modification on water and organic solvent nanofiltration using ceramic hollow fiber membrane. Ceram. Int. 2021, 47, 34020–34027.
- Madalosso, H.B.; Machado, R.; Hotza, D.; Marangoni, C. Membrane Surface Modification by Electrospinning, Coating, and Plasma for Membrane Distillation Applications: A State-of-the-Art Review. Adv. Eng. Mater. 2021, 23, 2001456.
- Hu, L.-H.; Tsai, Y.-T. Carbon Fiber Surface-Modified by Polymer Derived Ceramic Incorporated with Graphene to Strengthen the Mechanical and Electrochemical Properties of Ceramic-Carbon Fiber Composite. Compos. Sci. Technol. 2022, 221, 109294.
- Hu, L.-H.; Wang, Y.-K. Silicon Carbonitride Ceramic Surface-Modified Nanoporous Aluminum Alloy by Preceramic Polysilazane Precursor for Surface Strengthening. Mater. Sci. Eng. B 2021, 267, 115113.
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 2418–2421.
- Masuda, Y.; Seo, W.S.; Koumoto, K. Arrangement of Nanosized Ceramic Particles on Self-Assembled Monolayers. Jpn. J. Appl. Phys. 2000, 39, 4596–4600.
- Li, J.; Ji, S.; Zhang, G.; Guo, H. Surface-Modification of Poly(dimethylsiloxane) Membrane with Self-Assembled Monolayers for Alcohol Permselective Pervaporation. Langmuir 2013, 29, 8093–8102.
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998.
- Kossovsky, N.; Gelman, A.; Sponsler, E.E.; Hnatyszyn, H.; Rajguru, S.; Torres, M.; Pham, M.; Crowder, J.; Zemanovich, J.; Chung, A.; et al. Surface-Modified Nanocrystalline Ceramics for Drug Delivery Applications. Biomaterials 1994, 15, 1201–1207.
- Kühnle, A. Self-assembly of organic molecules at metal surfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 157–168.
- Patil, N.; Camacho, A.C.; Mishra, N.K.; Singhla, P.; Sweeney, C.B.; Saed, M.A.; Radovic, M.; Green, M.J. Radio Frequency and Microwave Heating of Preceramic Polymer Nanocomposites with Applications in Mold-Free Processing. Adv. Eng. Mater. 2019, 21, 1900276.
More
