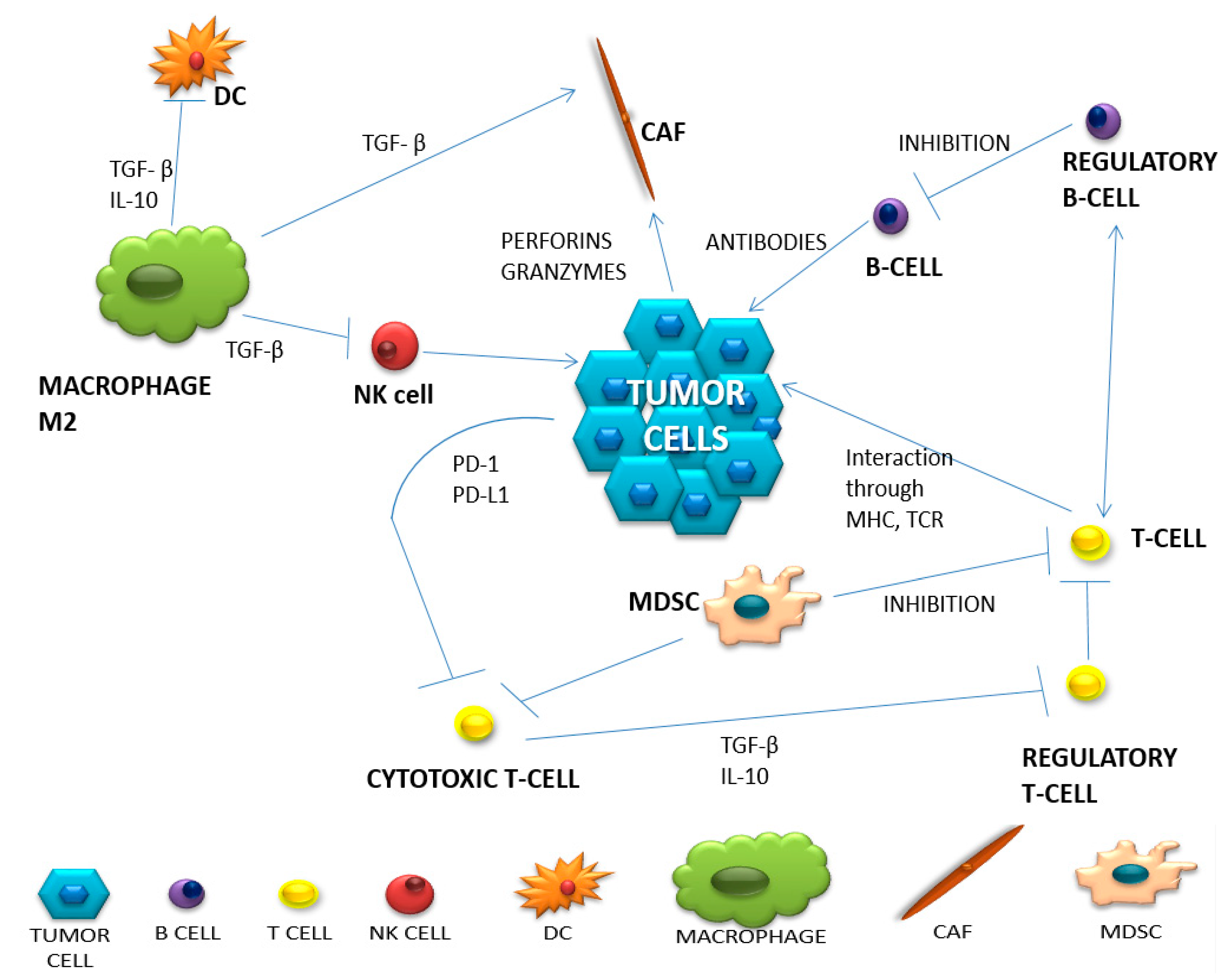
The tumor microenvironment is a complex network of various interactions between immune cells and non-cellular components such as the extracellular matrix, exosomes and interleukins. Moreover, tumor heterogeneity and its constant modification may alter the immunophenotype and become responsible for its resistance regarding the therapies applied However, it should be remembered that in a strongly immunosuppressive neoplastic microenvironment, the immune system cells undergo reprogramming and most often cease to fulfill their original function. Therefore, understanding what happens within the tumor microenvironment, and which mechanisms are responsible for tumor development and progression should let us know how cancer could protect itself against the immune system.
- tumor microenvironment
- immunophenotype
- T lymphocytes
1. Diversity of Intercellular Interactions in the Tumor Microenvironment
| Cell Type | Natural Function | Function in Tumor Microenvironment | Produced Substances | |
|---|---|---|---|---|
| of Anti-Tumor Activity | of Pro-Tumor Activity | |||
| HELPER T CELLS | Th1-stimulates dendritic and NK cells. Attracts T lymphocytes. |
Th1- blocked by IL-4, IL-10, TGF-β, Treg, Th2, M2 Th2-. Inhibition of Th1. Stimulation of M2 population of macrophages. |
TNF-α, IL-12, IL-17,IL-18, IL-21, IL-27. |
Th17 lymphocytes can transform into Treg lymphocytes. |
| REGULATORY T CELLS | Protection against autoimmunity from autoreactive T lymphocytes. | Stimulating immune tolerance. Immunosuppression. Inhibition of the immune response. |
IL-10, TGF-β, adenosine, PGE2, IL-35. | TGF-β, IL-2, IL-10, IL-35. |
| CYTOTOXIC T CELLS | Cytotoxic. Stimulation of other immune cells to infiltrate the tumor. |
Inhibition of the cytotoxic function by binding to PD-L1. | Perforins. Granzymes IL-2, TNF-α, IFN-γ. |
------ |
| MACROPHAGES | Destruction, phagocytosis of abnormal cells. Inducing inflammation. |
TAM2: Protumor. Inhibition of the inflammatory process. |
IFN-γ, IL-12, GM-CSF. | IL-10, TGF-β, EGF, FGF, VEGF, MMP CCL2, CCL5, CCL3, CCL8, CCL22. |
| NEUTROPHILS | Phagocytosis. ADCC. Stimulation of CD8 + lymphocytes, NK cells. |
N1: Phagocytosis. Stimulation of apoptosis N2: Angiogenesis. Stimulation of the inflammatory process in the tumor. |
TNF-α, IFN- γ. |
TGF-β, MPO, MMP9, HGF, VEGF |
| B CELLS | Presenting antigens. Complement activation. Antibody production. |
Supporting angiogenesis. -inhibition of anti-cancer activities. Stimulation of Treg. |
IL-2. | IL-10, TGF-β. |
| CANCER ASSOCIATED FIBROBLASTS (CAF) | -------- | Secreting growth factors. Causing inflammation. |
-------- | CXCL1, CXCL2, CXCL3, CXCL12, CCL2, CCL5, CCL17, IL-8, GM-CSF TGF-β, IL-6, exosomes, HGF, IGF, CTGF. |
| NATURAL KILLER CELLS (NK) | Cytotoxic. Immune supervision. Stimulation of T and DC lymphocytes. |
-------- | IL-2, IL-6, 12, 15, IFN-γ, TNF-α, GM-CSF, CCL-5. | -------- |
| DENDRITIC CELLS (DC) | Presentation of antigens. Influences the differentiation of helper and regulatory lymphocytes. Cytotoxic function. |
Presence on the surface of PD-L1, Impaired antigen presentation, maturation and tumor infiltration. |
IL-6, IL-8, IL-12, IL-15. |
-------- |
| CANCER ASSOCIATED ADIPOCYTES (CAA) | Production and storage of simple fats (triglycerides). | Secretion of adipokines. They cause changes in the metabolism of cancer cells and remodel the ECM. |
-------- | Adipokines: leptin, hepatocyte growth factors IL-1β. |
| MYELOID-DERIVED SUPPRESSOR CELLS (MDSC) | -------- | Suppression of the immune response. | -------- | IL-4, CCL3, CCL4, CCL5, PGE2, NO. |
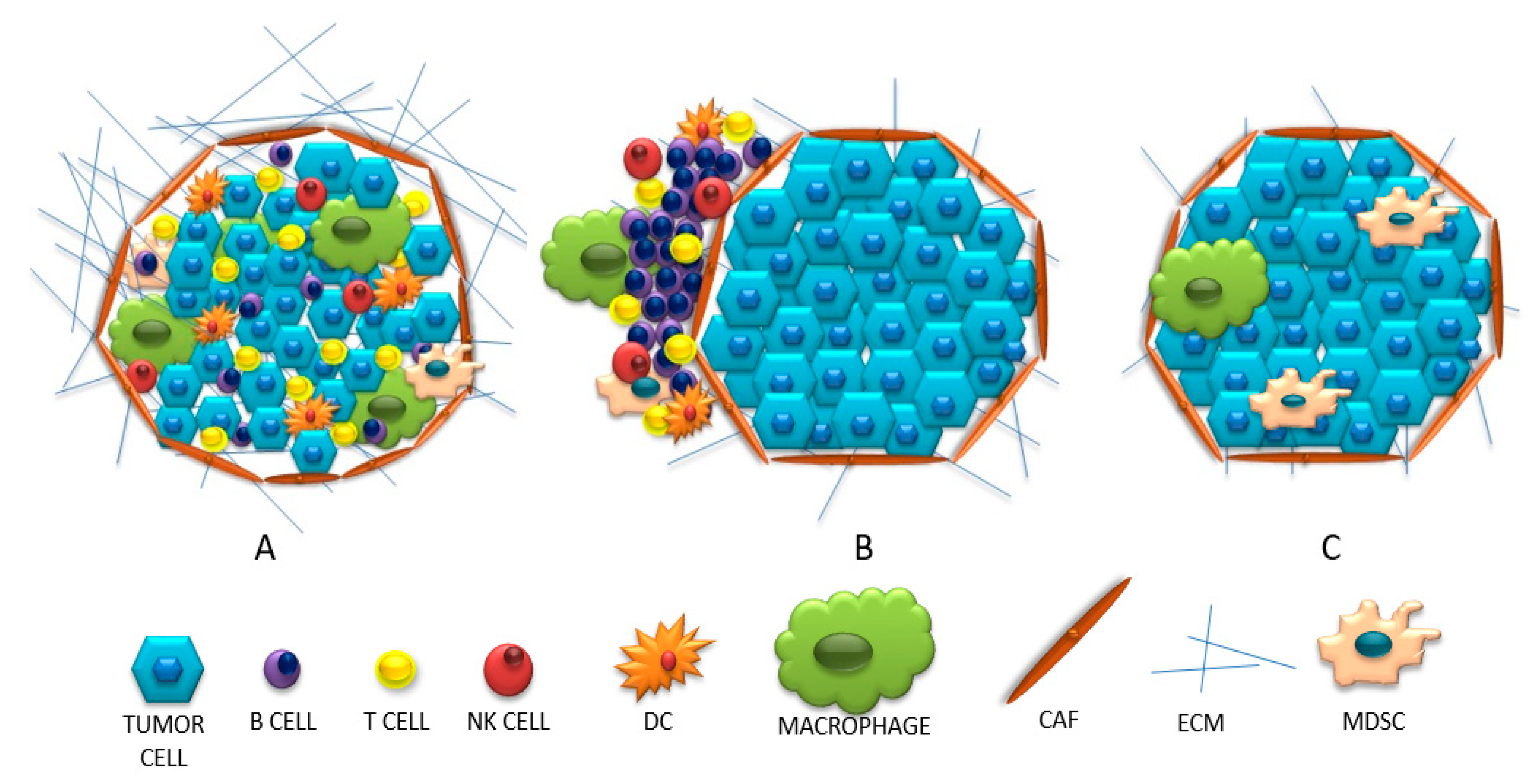
2. Three Categories of Tumor Microenvironments Based on Their Immunophenotype
References
- Gołąb, J.; Jakóbisiak, M.; Lasek, W.; Stokłosa, T. Immunologia; PWN: Warsaw, 2017; pp. 1–5.
- Farc, O.; Cristea, V. An overview of the tumor microenvironment, from cells to complex networks (Review). Exp. Ther. Med. 2020, 21, 1–13.
- Deng, S.; Clowers, M.J.; Velasco, W.V.; Ramos-Castaneda, M.; Moghaddam, S.J. Understanding the Complexity of the Tumor Microenvironment in K-ras Mutant Lung Cancer: Finding an Alternative Path to Prevention and Treatment. Front. Oncol. 2020, 9, 1–24.
- Pallegar Nikitha, K. CSL. Adipocytes in the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–13.
- Iwahori, K. Cytotoxic CD8+ Lymphocytes in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2018, 1224, 53–62.
- Lee, J.; Lozano-Ruiz, B.; Yang, F.M.; Fan, D.D.; Shen, L.; González-Navajas, J.M. The Multifaceted Role of Th1, Th9, and Th17 Cells in Immune Checkpoint Inhibition Therapy. Front. Immunol. 2021, 12, 625667.
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133.
- Galli, F.; Aguilera, J.V.; Palermo, B.; Markovic, S.N.; Nisticò, P.; Signore, A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 89.
- Jeske, S.S.; Weissinger, S.E.; Veit, J.A.; Brunner, C.; Huber, U.; Theodoraki, M.N.; Doescher, J. Treatment-induced changes of lymphocyte subsets in patients with adenoid cystic carcinoma of the head and neck. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 1465–1473.
- Massimo, A.; Jun-Li, L.; Sergei, G.; Sergei, N.; Michael, K. Prostate Cancer—NCCN Evidence Blocks. Version 2.2017. Am. Cancer Soc. 2017, 464, 302–305.
- Borros, A. Tumor microenvironment. Medicina 2020, 56, 1–21.
- Tsou, P.; Katayama, H.; Ostrin, E.J.; Hanash, S.M. The emerging role of b cells in tumor immunity. Cancer Res. 2016, 76, 5591–5601.
- Qing, Z.; Jiacheng, B.; Xiaodong, Z.; Yongyan, C.; Hua, W.; Wenyong, W.; Zhengguang, W.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732.
- Kim, J.; Bae, J.S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016, 2016, 6058147.
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Klein, A.M. Single cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 2019, 50, 1317–1334.
- Verneau, J.; Sautés-Fridman, C.; Sun, C.M. Dendritic cells in the tumor microenvironment: Prognostic and theranostic impact. Semin. Immunol. 2020, 48, 101410.
- Wu Lingyun, S.S.; Singh Rakesh, K. Neutrophils in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 41–52.
- Ishii, G.; Ochiai, A.; Neri, S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv. Drug Deliv. Rev. 2016, 99, 186–196.
- Monteran, L.; Erez, N. The dark side of fibroblasts: Cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol. 2019, 10, 1–15.
- LeBleu, V.S.; Kalluri, R. A peek into cancer-associated fibroblasts: Origins, functions and translational impact. DMM Dis. Model Mech. 2018, 11, dmm029447.
- Wu, Q.; Li, B.; Sun, S.; Sun, S. Unraveling Adipocytes and Cancer Links: Is There a Role for Senescence? Front. Cell Dev. Biol. 2020, 8, 1–7.
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 1–15.
- Wu, Q.; Li, B.; Li, J.; Sun, S.; Yuan, J.; Sun, S. Cancer-associated adipocytes as immunomodulators in cancer. Biomark. Res. 2021, 9, 2.
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220.
- Kumar, V.; Cheng, P.; Condamine, T.; Mony, S.; Languino, L.R.; McCaffrey, J.C.; Gabrilovich, D.I. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Physiol. Behav. 2017, 176, 139–148.
- De Guillebon, E.; Dardenne, A.; Saldmann, A.; Séguier, S.; Tran, T.; Paolini, L.; Tartour, E. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int. J. Cancer 2020, 147, 1509–1518.
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618.
- van der Woude, L.L.; Gorris, M.A.J.; Halilovic, A.; Figdor, C.G.; de Vries, I.J.M. Migrating into the Tumor: A Roadmap for T Cells. Trends Cancer 2017, 3, 797–808.
- Ros, X.R.; Vermeulen, L. Turning Cold Tumors Hot by Blocking TGF-β. Trends Cancer 2018, 4, 335–337.
