Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 3 by Conner Chen.
Ferroptosis, which has been widely associated with many diseases, is an iron-dependent regulated cell death characterized by intracellular lipid peroxide accumulation. It exhibits morphological, biochemical, and genetic characteristics that are unique in comparison to other types of cell death. The course of ferroptosis can be accurately regulated by the metabolism of iron, lipids, amino acids, and various signal pathways.
- ferroptosis
- chronic diseases
- food-borne active ingredients
1. Role of Ferroptosis in Chronic Diseases
Ferroptosis has recently become a hotspot of research focus in the field of disease prognosis and therapy, with numerous studies reporting that it regulates the occurrence and progress of multiple disorders (Figure 12). Here, the latest progress in ferroptosis research and its association with various diseases are summarized.
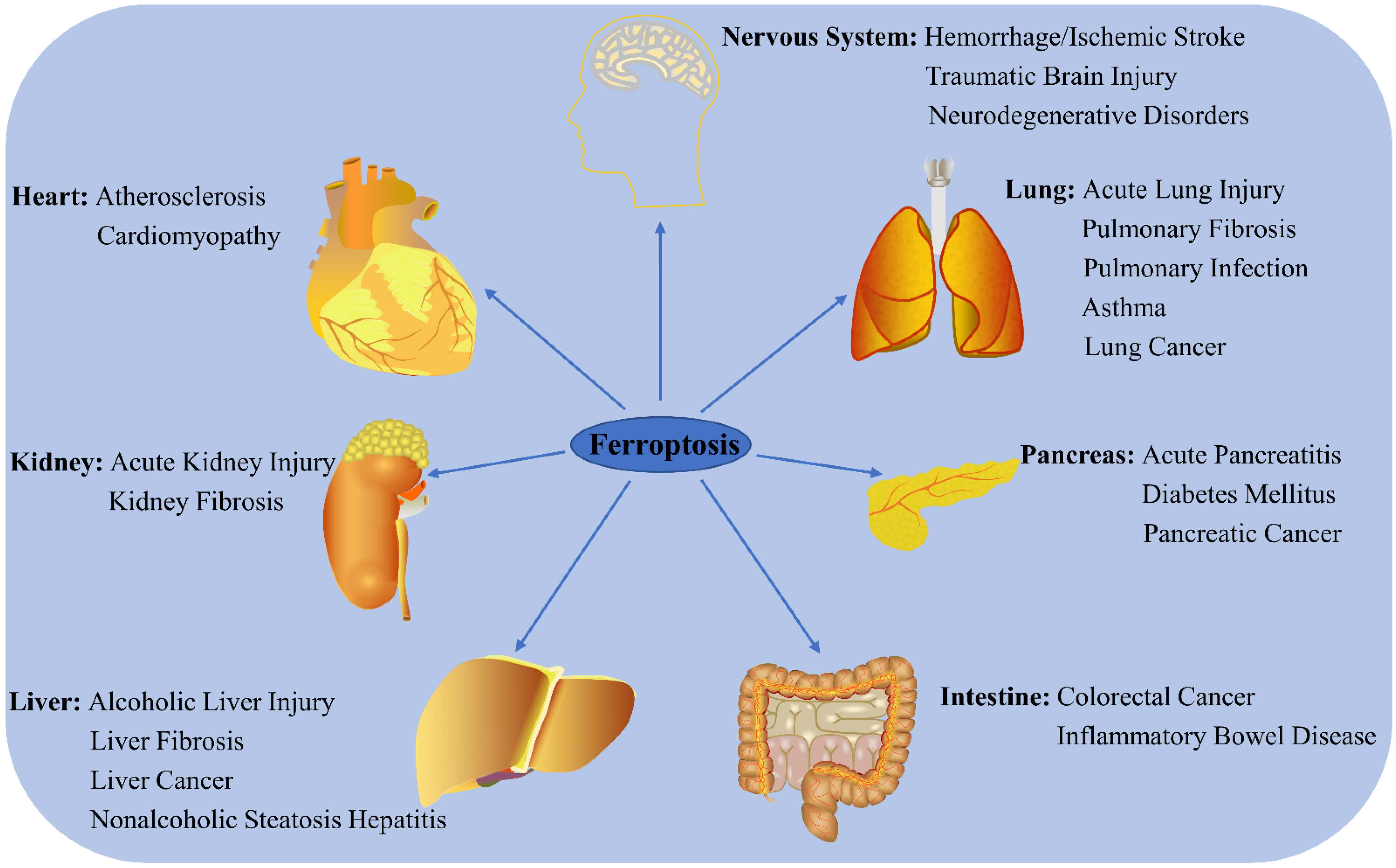
Figure 12. Role of ferroptosis in chronic diseases. Ferroptosis participates in the regulation of many system disorders, including nervous system diseases, cardiovascular diseases, liver diseases, kidney diseases, lung diseases, pancreatic diseases, and intestinal diseases.
1.1. Cancers
1.1.1. Lung Cancer
Lung cancer is one of the most common causes of cancer-related deaths worldwide. Some key regulators, including KRAS, TP53, Nrf2, YAP, NFS1, STYK1, LSH, RNF113A, and non-coding RNA, are reported to participate in ferroptosis regulation [1]. KRAS mutant lung cancer cells are vulnerable to the ferroptosis induced by SLC7A11 inhibition [2]. P53 also inhibits SLC7A11 expression and cystine uptake, consequently inducing ferroptosis [3]. The activation of Nrf2 negatively regulates ferroptosis by up-regulating various target genes, such as HO-1. Acetaminophen (APAP) sensitizes non-small-cell lung cancer (NSCLC) to erastin-mediated ferroptosis by negatively regulating the Nrf2/HO-1 signaling pathway [4]. Curcumin triggers ferroptosis in NSCLC by activating autophagy [5]. DHA induces lung cancer cell ferroptosis via the inactivation of the PRIM2/SLC7A11 axis [6]. Orlistat promotes lung cancer cell ferroptosis by reducing GPX4 expression and inducing lipid peroxidation [7]. A pure compound extracted from danshen, dihydroisotanshinone I, suppresses the growth of lung cancer cells by triggering both ferroptosis and apoptosis [8]. The artemisinin derivatives artesunate and DHA induce ROS-dependent apoptosis/ferroptosis in NSCLC cells [9]. MiR-27a-3p promotes NSCLC through SLC7A11-mediated-ferroptosis [10], while MiR-302a-3p induces the ferroptosis of NSCLC cells by targeting FPN [11].
1.1.2. Liver Cancer
Liver cancer is the sixth most common malignancy and the third primary cause of cancer-related deaths worldwide [12]. Ferroptosis plays an important role in the regulation of hepatocellular carcinoma (HCC) (Figure 23). Sorafenib, which is a multikinase inhibitor used widely in the treatment of advanced HCC, induces ferroptosis by inhibiting SLC7A11; however, activation of the p62-Keap1-Nrf2 pathway suppresses ferroptosis in HCC [13]. Erastin and sorafenib induce ferroptosis by inhibiting Nrf2 expression and activity in HCC [14]. Sorafenib also decreases retinoblastoma protein levels, thereby increasing ferroptosis [15], while the inhibition of metallothionein enhances sorafenib-induced ferroptosis in HCC [16]. Haloperidol strongly promotes erastin- and sorafenib-induced ferroptosis by increasing Fe2+ levels and lipid peroxidation in HCC [17]. Ketamine induces ferroptosis via the inhibition of lncRNA PVT1 and GPX4 in liver cancer cells [18]. DHA induces ferroptosis by activating unfolded protein response and upregulating CHAC1 expression in primary liver cancer cells [19]. MiR-214-3p promotes erastin-mediated ferroptosis by decreasing ATF4 expression in hepatoma cells [20]. Artesunate increases the sensitivity of HCC to sorafenib by inducing ferroptosis [21], while O-GlcNAcylation sensitizes liver cancer to RSL3-induced ferroptosis through the YAP/TFRC pathway [22].
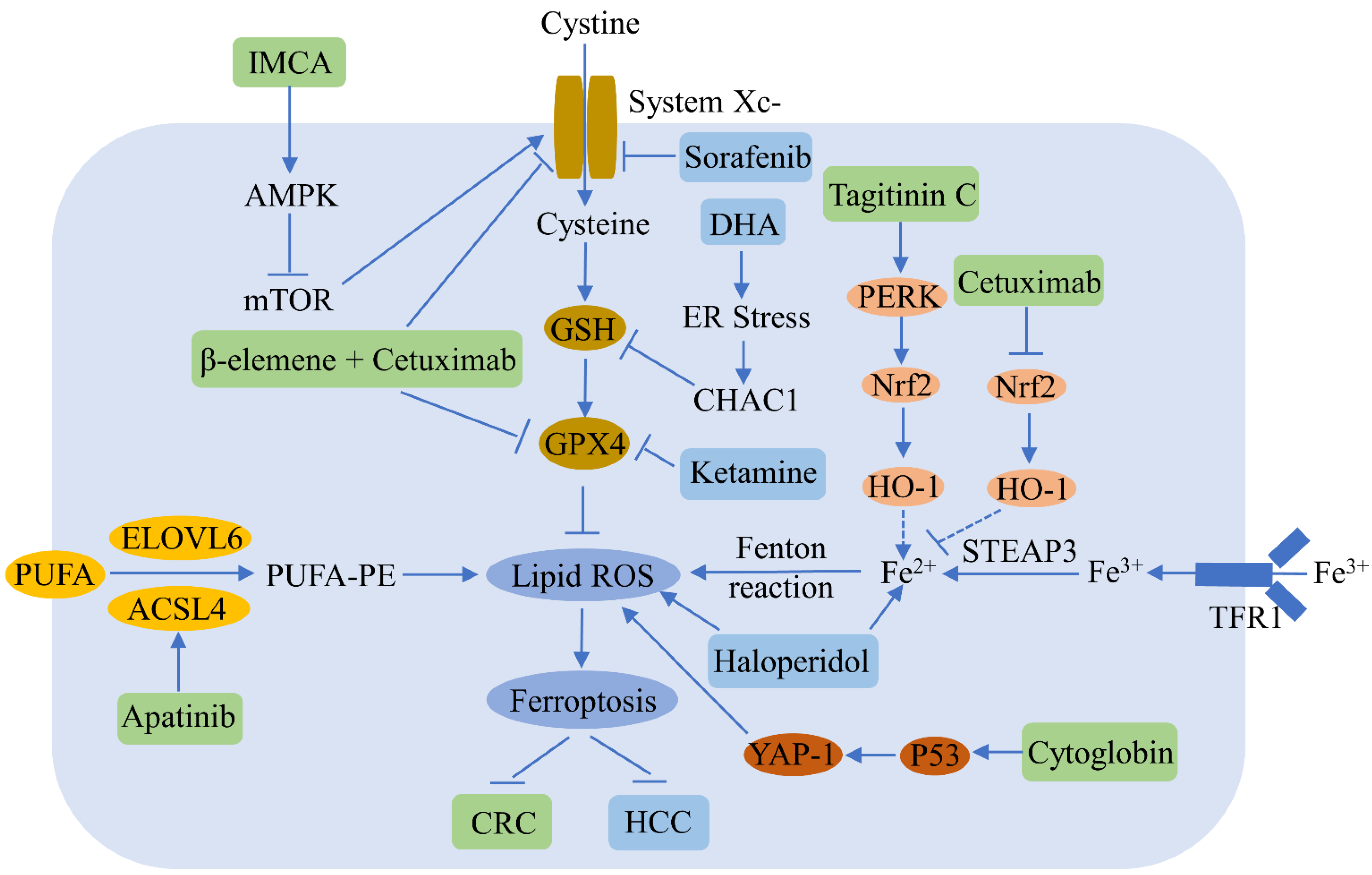
Figure 23. Ferroptosis regulators and pathways in hepatocellular carcinoma (HCC) and colorectal cancer (CRC). Ferroptosis can be a negative regulator of HCC and CRC. Various compounds can inhibit HCC and CRC growth by inducing ferroptosis. The blue and green patterns correspond to regulators for HCC and CRC, respectively. Sorafenib induces HCC ferroptosis by inhibiting SLC7A11. Dihydroartemisinin (DHA) triggers HCC ferroptosis by activating unfolded protein response and upregulating CHAC1. Ketamine increases HCC ferroptosis by inhibiting GPX4. Haloperidol promotes ferroptosis via the increase of Fe2+ levels and lipid peroxidation in HCC. IMCA induces CRC ferroptosis by inhibiting SLC7A11. β-elemene and cetuximab combined treatment results in CRC ferroptosis by downregulating GPX4 and SLC7A11. Apatinib enhances CRC ferroptosis by up-regulating ELOVL6/ACSL4 signaling. Tagitinine C induces CRC ferroptosis via the up-regulation of the PERK-Nrf2-HO-1 signaling pathway. Cetuximab increases CRC ferroptosis by downregulating the Nrf2/HO-1 pathway. Cytoglobin increases CRC ferroptosis through the upregulation of p53 and YAP1.
1.1.3. Colorectal Cancer
Recent studies have reported that ferroptosis participates in the regulation of colorectal cancer (CRC), a common malignancy of the digestive system (Figure 23). RSL3 is known to induce CRC ferroptosis by inactivating GPX4 and producing ROS [23]. Tagitinine C, a natural product, acts synergistically with erastin to induce ferroptosis in CRC cells via the PERK-Nrf2-HO-1 signaling pathway [24]. The benzopyran derivative IMCA induces ferroptosis by downregulating SLC7A11 and the AMPK/mTOR pathway in CRC [25]. Inhibiting the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway triggers ferroptosis and sensitizes CRC to oxaliplatin [26]. Cetuximab increases the ferroptosis of KRAS mutant CRC, induced by RSL3, by inhibiting the Nrf2/HO-1 signaling pathway [27]. A combined treatment of β-elemene and cetuximab induces KRAS mutant CRC cell ferroptosis via the downregulation of GPX4 and SLC7A11 [28], and the inhibition of SLC7A11 induces the ferroptosis of CRC stem cells [29]. Apatinib enhances ferroptosis in CRC cells by upregulating ELOVL6/ACSL4 signaling [30]. Cytoglobin sensitizes the CRC cells to RSL3 and erastin through the upregulation of p53 and YAP1 [31]. Andrographis enhances the effect of 5-fluorouracil (5FU) against CRC by inducing ferroptosis and inhibiting β-catenin/Wnt-signaling pathways [32].
1.1.4. Breast Cancer
Breast cancer is the most common cancer among women. Sulfasalazine has been found to trigger breast cancer cell ferroptosis via the repression of GPX4 and SLC7A11 expressions and an increase in TFR1 and DMT1 expressions, particularly in cells with low estrogen receptor (ER) expression [33]. The combined treatment of siramesine and lapatinib triggers breast cancer cell ferroptosis by increasing TF expression and reducing FPN expression [34]. Metformin triggers ferroptosis via a reduction in the UFMylation of SLC7A11 in breast cancer [35]. Curcumin promotes the ferroptosis of breast cancer cells by increasing lipid ROS levels, lipid peroxidation end-product MDA accumulation, and intracellular free iron levels [36]. Targeted exosome-encapsulated erastin promotes the ferroptosis of triple-negative breast cancer (TNBC) cells [37]. The GSK3β/Nrf2 signaling pathway strengthens the ferroptosis of breast cancer induced by erastin [38]. Inhibiting GPX4 increases gefitinib-induced ferroptosis in TNBC cells [39], while simvastatin induces ferroptosis in TNBC cells [40]. Metformin promotes ferroptosis via the up-regulation of miR-324-3p and down-regulation of GPX4 in breast cancer [41]. Lidocaine induces the ferroptosis of ovarian and breast cancers by increasing miR-382-5p and decreasing SLC7A11 [42], while ketamine induces ferroptosis by targeting the KAT5/GPX4 axis in breast cancer cells [43].
1.1.5. Ovarian Cancer
Ovarian cancers are prevalent female malignancies, seriously affecting women’s health and life quality in the world. Lidocaine promotes ferroptosis in ovarian and breast cancer cells by enhancing miR-382-5p and down-regulating SLC7A11 expression [42]. Stearoyl-CoA desaturase 1 (SCD1) suppresses ovarian cancer cell ferroptosis [44], while the inhibition of pharmaceutical SCD1 promotes ferroptosis in vitro and in vivo, and the combined treatment of SCD1 inhibitors and ferroptosis inducers significantly inhibits ovarian tumor growth [45]. SNAI2 knockdown promotes ferroptosis in ovarian cancer [46]. Sodium molybdate induces the ferroptosis of ovarian cancer cells via labile iron elevation and GSH depletion [47]. Ferroptosis inducers increase the sensitivity of BRCA-proficient ovarian cancer cells to PARP inhibitor by repressing SLC7A11 [48]. Human serum incubated-superparamagnetic iron oxides promote ferroptosis via p53 overexpression in ovarian cancer cells [49]. Superparamagnetic iron oxide nanoparticles increase oxidative stress, reduce autophagy, and activate ferroptosis in ovarian cancer stem cells [49]. GALNT14 induces ferroptosis in ovarian cancer via the EGFR/mTOR pathway [50]. MAP30 protein from Momordica charantia and cisplatin synergistically induce ferroptosis in ovarian cancer [51].
1.1.6. Pancreatic Cancer
Pancreatic cancer, one of the most fatal of all cancers, is seen mainly in males and the older population (40–85 years) [52]. The inhibition of cytosolic aspartate aminotransaminase promotes pancreatic cancer cell ferroptosis by repressing mitochondrial metabolism and promoting a catabolic state [53]. The natural compound artesunate induces ferroptosis in pancreatic cancer cells [54]. Cysteine depletion promotes the ferroptosis of pancreatic tumor cells in mice [55]. Ruscogenin, a saponin found in the root of Ophiopogon japonicus, increases intracellular ferrous iron and ROS, thereby inducing ferroptosis in pancreatic cancer cells [56]. The abrogation of ADP ribosylation factor 6 promotes RSL3-induced ferroptosis in pancreatic cancer cells [57]. DHA acts synergistically with cisplatin to trigger ferroptosis in pancreatic ductal adenocarcinoma (PDAC) by regulating iron metabolism [58]. Combining artesunate with GRP78 inhibition facilitates ferroptosis in KRAS mutant PDAC [59]. Chrysin enhances pancreatic cancer sensitivity to gemcitabine by inducing autophagy-dependent ferroptosis via the targeting of human carbonyl reductase 1 [60]. Ponicidin suppresses pancreatic cancer growth by inducing ferroptosis [61], while piperlongumine induces ferroptosis in human pancreatic cancer cells by suppressing the gamma-glutamyl cycle and regulating the metabolism of PUFAs [62].
1.2. Nervous System Diseases
1.2.1. Stroke
Stroke is one of the leading causes of death and disability in the world. Selenium activates homeostatic transcription to protect neuron cells from ferroptosis and treat stroke [63]. ACSL4 enhances ischemic stroke by inducing neuronal ferroptosis-related brain injury and neuroinflammation [64], while the inhibition of ACSL4 improves neurological functioning after stroke via the suppression of ferroptosis [65]. Reducing NCOA4 inhibits the ferritinophagy-mediated ferroptosis of neurons, and thus can be used to treat ischemic stroke [66]. The hemin-induced hemorrhagic stroke model is one of classic neuronal ferroptosis [67]. Intracerebral hemorrhage (ICH) is a type of severe stroke, the pathology of which is closely related to ferroptosis. Curcumin nanoparticles inhibit ferroptosis and enhance ICH treatment [68], and baicalin can also suppress ferroptosis in ICH [69]. Tau-mediated iron export inhibits ferroptosis after ischemic stroke [70]. Supplementing lactoferrin reduces the ferroptosis of nerve cells after diabetic ICH [71], while crocin reduces ICH-induced neuronal ferroptosis by increasing Nrf2 expression and nuclear translocation [72]. Dauricine represses nerve cell ferroptosis and brain injury after ICH by up-regulating GPX4 expression [73], while pyridoxal isonicotinoyl hydrazone, a lipophilic iron-chelating agent, alleviates hemorrhage stroke by decreasing ferroptosis and inflammation [74].
1.2.2. Traumatic Brain Injury
Traumatic brain injuries (TBIs) occur worldwide and result in serious economic burden. Inhibiting ferroptosis reduces tissue damage and ameliorates long-term motor and cognitive function after TBI in mice [75]. Biomarkers of ferroptosis are increased after TBI; however, baicalein decreases neuronal ferroptosis and improves post-TBI outcome [76]. Ferritin H deletion in nerve cells abolishes the neuroprotection of melatonin against TBI-mediated ferroptosis [77]. Prokineticin-2 prevented neuronal cells from ferroptosis in a TBI model [78]. Polydatin alleviates TBI via the inhibition of ferroptosis [79]. MiR-212-5p attenuates neuronal ferroptosis after TBI by targeting PTGS2 [80]. Ruxolitinib was found to protect against neuronal ferroptosis in a mouse TBI model [81]. Ferristatin II, an iron uptake inhibitor, prevents neuronal ferroptosis after TBI [82], while tetrandrine ameliorates TBI by regulating autophagy to reduce ferroptosis [83].
1.2.3. Alzheimer’s Disease
Alzheimer’s disease (AD) is the most ordinary type of dementia, featuring β-amyloid and Tau protein accumulation and aggregation [84]. FPN loss induces ferroptosis and promotes memory impairment in AD [85]. NADPH oxidase 4 promotes astrocytes ferroptosis via oxidative-stress-induced lipid peroxidation in AD [86]. Mitochondrial aldehyde dehydrogenase reduces AD-induced cardiac anomalies by inhibiting ACSL4-mediated ferroptosis [87]. Tetrahydroxy stilbene glycoside was shown to improve AD in APP/PS1 mice via the inhibition of GPX-related ferroptosis [88], while eriodictyol improves cognitive disorder in APP/PS1 mice by suppressing ferroptosis via Nrf2 activation mediated by a vitamin D receptor [89]. In addition, forsythoside A alleviates AD by reducing Nrf2/GPX4 activation-induced ferroptosis [90].
1.2.4. Parkinson’s Disease
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by motor impairment. FTH1 was reported to suppress ferroptosis by impairing ferritinophagy in a 6-OHDA-induced PD model [91]. Activating the p62-Keap1-Nrf2 pathway inhibits the ferroptosis triggered by 6-OHDA in dopaminergic cells [92]. Thioredoxin-1 reduces ferroptosis in PD by up-regulating GPX4 and GSH [93]. Super-enhancer-driven sorting nexin 5 expression facilitated the ferroptosis of dopaminergic neurons in PD models [94]. MiR-335 enhanced ferroptosis by degrading FTH1 in in vitro and in vivo models of PD [95]. Moxibustion presents a neuroprotective function via anti-ferroptosis in PD [96][97]. Ferritinophagy-mediated ferroptosis involves in paraquat-induced neurotoxicity in PD [98].
1.2.5. Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant and fatal neurodegenerative disease resulting from abnormal cytosine-adenine-guanine repetition in the huntingtin gene [99], for which there is currently no effective treatment. A few ferroptotic characteristics have been observed in HD animal models and patients, such as iron accumulation, oxidative stress, GSH depletion, and reduced GPX activity [100], which suggests the participation of ferroptosis in the regulation of HD pathogenesis. Several ferroptosis regulators have also been found to take effect in HD models. For instance, Fer-1 suppressed oxidative lipid damage and ferroptosis in HD cellular models [101], while the intraventricular delivery of the iron chelator DFO led to motor phenotype improvement in R6/2 HD mice [102].
1.3. Metabolic Diseases
1.3.1. Cardiovascular Diseases
Cardiovascular diseases are those involving the heart and blood vessels, such as atherosclerosis, peripheral vascular diseases, and cerebrovascular diseases. Ferritin plays a key role in inhibiting cardiac ferroptosis and succedent heart failure, while cardiac ferritin H loss promotes cardiomyopathy by increasing SLC7A11-mediated ferroptosis [103]. Mitochondria-dependent ferroptosis plays an important role in doxorubicin-induced cardiomyopathy [104]. ENPP2, a lipid kinase participating in lipid metabolism, reduces erastin-induced ferroptosis in cardiomyocytes by regulating GPX4, ACSL4, and Nrf2 expression and increasing AKT signaling [105]. The inhibition of ferroptosis alleviates atherosclerosis by reducing lipid peroxidation and endothelial dysfunction [106]. Rapamycin plays a key role in reducing excess iron and ferroptosis in cardiomyocytes [107]. MSC exosomes derived from human umbilical cord blood inhibit ferroptosis and attenuate myocardial injury, possibly by inhibiting the expression of DMT1 by miR-23a-3p [108]. GPX4 down-regulation during myocardial infarction results in ferroptosis in cardiomyocytes [109]. TRIM21 ablation alleviates cardiotoxicity of the chemotherapeutic agent doxorubicin by suppressing ferroptosis [110].
1.3.2. Diabetes Mellitus
Diabetes mellitus is classified as either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM), the latter of which affects approximately 90% to 95% of all diabetes mellitus patients. Quercetin potentially alleviates T2DM by decreasing pancreatic iron deposition and pancreatic β cell ferroptosis [111]. SLC40A1 mediates ferroptosis and cognitive dysfunction in T1DM [112]. SIRT3 deficiency suppresses autophagy-mediated ferroptosis by suppressing AMPK-mTOR pathway activation and enhancing GPX4 levels, thus providing a potential therapeutic approach for gestational diabetes mellitus [113]. Melatonin reduces ferroptosis levels by activating the Nrf2/HO-1 signaling pathway in T2DM osteoporosis [114]. Ferroptosis participates in the development of diabetic nephropathy (DN); however, Nrf2 upregulation inhibits ferroptosis and delays the progression of DN [115]. Ferroptosis might enhance diabetic renal tubular injury via the HIF-1α/HO-1 pathway [116]. HMOX1 upregulation enhances ferroptosis in diabetic atherosclerosis [117]. Autophagy inhibition leads to the ferroptosis of cardiomyocytes and worsens diabetic cardiomyopathy development in mice by targeting Nrf2 [118]. DFO treatment alleviates poststroke cognitive impairment in diabetes by inhibiting ferroptosis [119]. Caveolin-1 reduces diabetes-related cognitive disorder by regulating neuronal ferroptosis-mediated mitochondrial homeostasis [120].
1.3.3. Liver Diseases
Increasing evidence indicates that the suppression of ferroptosis may slow the development of several liver diseases, including alcoholic liver injury, nonalcoholic steatosis hepatitis (NASH), and fibrosis [121]. Intestinal SIRT1 deficiency attenuates alcoholic liver injury via the mitigation of hepatic ferroptosis in mice [122]. Ginkgolide B, a primary ingredient of Ginkgo biloba extracts, alleviates nonalcoholic fatty liver disease in obese mice by inhibiting ferroptosis, possibly through the Nrf2 signaling pathway [123]. Ferroptosis inhibitors reduce methionine/choline-deficient diet-induced NASH by inhibiting liver lipotoxicity [124]. (+)-Clausenamide, an active alkaloid isolated from the leaves of Clausena lansium (Lour.) Skeels, alleviates drug-induced liver injury by suppressing hepatocyte ferroptosis. The radical oxidation of n-6 PUFAs promotes ferroptosis and APAP-induced acute liver failure [125]. Ferroptosis plays a dual role in liver fibrosis. Some evidence suggests the pathogenic role of ferroptosis in iron-overload-induced liver fibrosis, and inhibiting ferroptosis potently prevents liver fibrosis. Hepatic TF plays a role in maintaining liver function and preventing ferroptosis-induced liver fibrosis [126]. Artesunate relieves liver fibrosis via downregulation of the ferroptosis signaling pathway [127]. Fibroblast growth factor 21 reduces iron-overload-induced liver injury and fibrosis by suppressing ferroptosis [128]. Activation of hepatic stellate cells (HSCs); that is, transdifferentiation into matrix-producing myofibroblasts, is considered the central driver of hepatic fibrosis [129]. Recent studies demonstrate the potential of inducing ferroptosis in HSCs as a therapeutic strategy designed to alleviate the development of liver fibrosis. Artemether relieves carbon-tetrachloride-induced liver fibrosis and inhibits HSCs activation through p53-dependent ferroptosis induction [130]. Sorafenib suppresses liver fibrosis by inducing HSCs ferroptosis via the inactivation of the HIF-1α/SLC7A11 pathway [131]. Moreover, certain regulators of ferroptosis in HSCs, including p53 [130], ELAV-like protein 1 (ELAVL1) [132], and zinc finger protein 36 (ZFP36) [133], have been reported as potential targets for the treatment of liver fibrosis.
1.3.4. Kidney Diseases
Ferroptosis has recently been associated with diverse kidney diseases, including acute kidney injury (AKI) and polycystic kidney disease. Quercetin reduces AKI by suppressing ferroptosis [134], while nuciferine also reduces folic-acid-induced AKI via the suppression of ferroptosis [135], and the inactivation of ferroptosis regulator GPX4 results in AKI in mice [136]. Inhibiting ferroptosis attenuates AKI in rats with severe acute pancreatitis [137], while the silencing of miR-182-5p and miR-378a-3p reduces ischemia/reperfusion-induced renal injury in rats by suppressing ferroptosis [138]. The inhibition of ferroptosis by XJB-5-131 reduces renal tubular cell injury in kidney diseases [139]. Targeted inhibition of the circadian clock components Rev-erb-α/β suppresses ferroptosis to reduce folic acid-induced AKI [140]. Dimethyl fumarate inhibits ferroptosis to attenuate AKI by targeting Nrf2 [141]. Fenofibrate inhibits ferroptosis by upregulating Nrf2, thereby resisting DN progression [115]. Activating the vitamin D receptor attenuates cisplatin-induced AKI by repressing ferroptosis partially through GPX4 trans-regulation [142]. Legumain enhances tubular ferroptosis via the activation of chaperone-mediated GPX4 autophagy in AKI [143]. Tocilizumab mimotopes reduce kidney injury and fibrosis by blocking IL-6 signaling and ferroptosis [144]. ACSL4 deficiency alleviates ferroptosis-mediated AKI [145].
1.3.5. Lung Diseases
Recent studies have suggested that ferroptosis plays an important role in a number of lung diseases, including acute lung injury (ALI), chronic obstructive pulmonary disease, pulmonary fibrosis, pulmonary infection, and asthma. Fer-1 relieves LPS-induced ALI by suppressing ferroptosis [146]. Nrf2 inhibits ferroptosis and alleviates ALI by increasing SLC7A11 and HO-1 expression [147]. The inhibitor of apoptosis-stimulating protein of p53 reduces IIR-ALI via the suppression of ferroptosis [148]. Panaxydol attenuates LPS-induced ALI by up-regulating the Keap1-Nrf2/HO-1 pathway to inhibit ferroptosis [149]. Wedelolactone mitigates acute pancreatitis associated with lung injury by increasing GPX4 to suppress pyroptosis and ferroptosis [150]. Nrf2 alleviates seawater-drowning-induced ALI by suppressing ferroptosis [151]. Nrf2 and STAT3 reduce IIR-ALI by decreasing SLC7A11-mediated ferroptosis [152]. Melatonin reduces PM2.5-induced lung injury through the suppression of lung epithelial cell ferroptosis via Nrf2 activation [153]. Sevoflurane reduces LPS-induced ALI by repressing ferroptosis via the upregulation of HO-1 expression [154]. Obacunone reduces LPS-induced ALI by suppressing ferroptosis via the increase of Nrf2-dependent antioxidant responses [155]. Hydrogen sulfide reduces ferroptosis and activates autophagy by downregulating mTOR signaling in sepsis-induced ALI [156]. The inhibition of ACSL4 mitigates ferroptosis in ischemia/reperfusion-induced lung injury by decreasing lipid peroxidation and increasing the GSH and GPX4 levels [157].
1.4. Inflammatory Bowel Diseases
Inflammatory bowel disease is a chronic relapsing disease mainly affecting the intestinal tract, and includes ulcerative colitis (UC) and Crohn’s disease (CD). Ferroptosis participates in intestinal epithelial cell death in UC [158]. The suppression of ferroptosis ameliorates DSS-induced UC by suppressing the Nrf2/HO-1 signaling pathway [159]. Curculigoside protects against ferroptosis in UC by inducing GPX4 [160]. Fer-1 reduces TNBS-induced colitis by inhibiting ferroptosis [161]. Furin inhibits the DSS-induced ferroptosis of epithelial cells and reduces experimental colitis via Nrf2-GPX4 signaling pathway activation [162]. Astragalus polysaccharide was shown to inhibit ferroptosis in a murine model of experimental colitis and human Caco-2 cells by blocking the Nrf2/HO-1 pathway [163]. Dietary lipids are a trigger of GPX4-restricted enteritis resembling CD [164].
2. Regulatory Effects of Food-Borne Active Ingredients on Ferroptosis
A large number of studies have reported that food-borne active ingredients, such as polyphenols, can regulate ferroptosis (Figure 34).
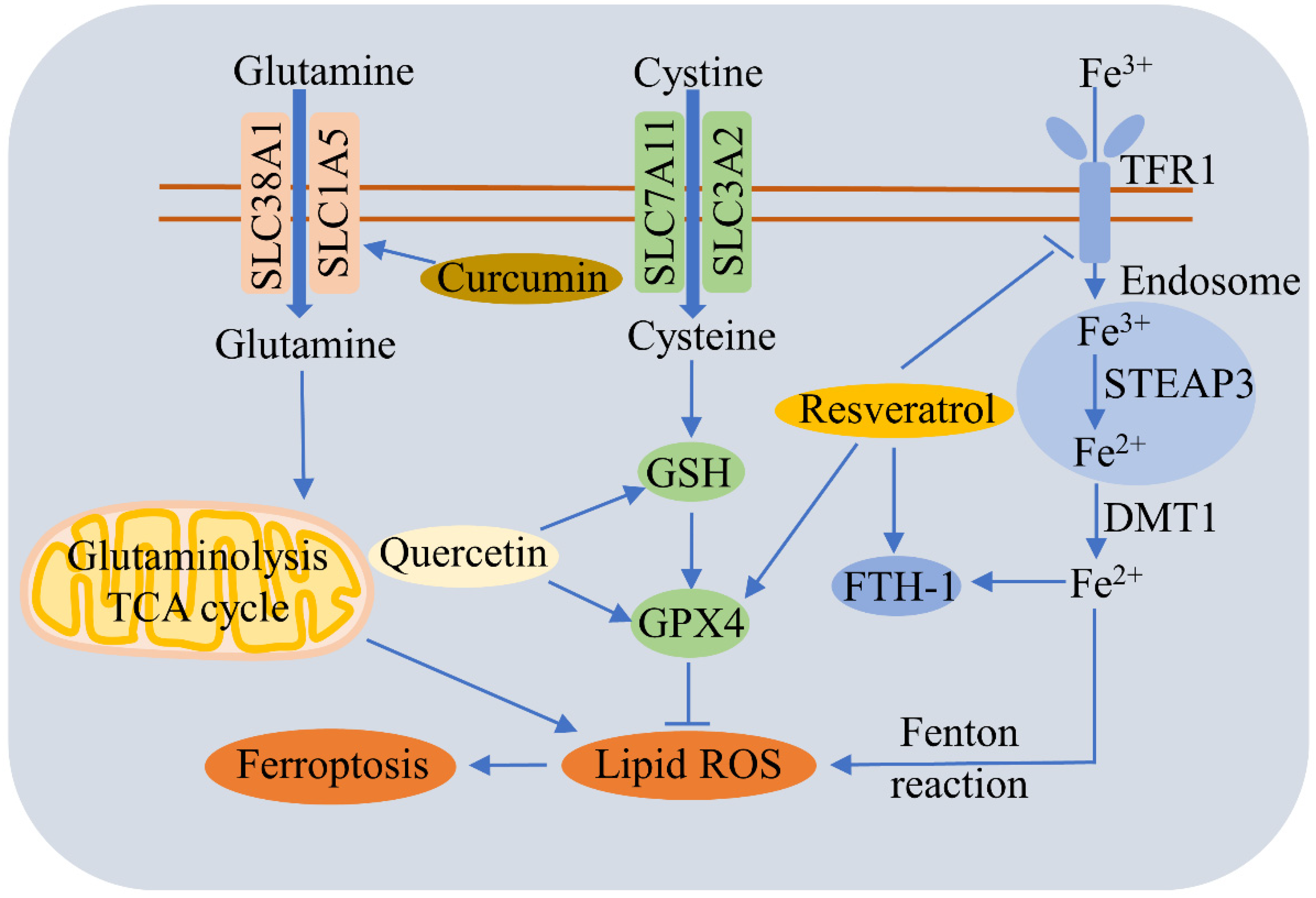
Figure 34. Regulatory effects of food-borne active ingredients on ferroptosis. This schematic diagram shows the regulation pathways of ferroptosis by polyphenols such as quercetin, curcumin, and resveratrol. Curcumin triggers ferroptosis by promoting SLC1A5-mediated glutamine uptake. Quercetin alleviates ferroptosis by upregulating GSH and GPX4. Resveratrol reduces ferroptosis by decreasing TfR1 expression and increasing the expressions of FTH1 and GPX4.
Quercetin (QCT), a natural flavonoid found commonly in fruits and vegetables, alleviates T2DM by inhibiting the ferroptosis of pancreatic β cells via the upregulation of GSH and GPX4, and by increasing mitochondria membrane-associated protein VDAC2, which functions as an antioxidant [111]. QCT also reduces AKI by repressing ferroptosis via a reduction in MDA and lipid ROS levels and an increase in GSH [134]. QCT can suppress the erastin-induced ferroptosis of bone-marrow-derived mesenchymal stem cells, probably via the antioxidant pathway [165]. QCT triggers p53-mediated cancer cell ferroptosis by promoting lysosome-dependent ferritin degradation and ROS generation [166]. Dihydroquercetin reverses cigarette-smoke-induced ferroptosis in the pathogenesis of chronic obstructive pulmonary disease by up-regulating the Nrf2-dependent pathway [167].
Gallic acid (GA) is a natural polyhydroxy phenolic compound seen in various foods, such as edible mushrooms, fruits, and vegetables. GA triggers cancer cell death via the activation of apoptotic, ferroptotic, and necroptotic pathways [168]. Preirradiation therapy followed by GA treatment inhibits the survival of cancer cells more effectively than GA treatment alone via the apoptosis and ferroptosis cell death mechanisms [169].
Curcumin, a polyphenol compound extracted from the turmeric plant, enhances the treatment effect of NSCLC by activating autophagy-dependent ferroptosis [5]. Curcumin decreases rhabdomyolysis-related renal damage by reducing ferroptosis, and mechanistic studies have shown that curcumin downregulates the TLR4/NF-κB axis and activates HO-1 [170]. Since curcumin nanoparticles suppress ferroptosis, they can be used to strengthen the treatment of ICH [68]. Curcumin also induces the ferroptosis of breast cancer cells by upregulating SLC1A5 [36].
Epigallocatechin gallate (EGCG), a major polyphenol in green tea, protects against radiation-induced intestinal injury by scavenging ROS and repressing apoptosis and ferroptosis via the Nrf2 signal pathway [171]. EGCG pretreatment reduces doxorubicin cardiotoxicity-induced ferroptosis by increasing AMPKα2 and promoting adaptive autophagy [172]. Apigenin is a flavonoid found in green leafy herbs and vegetables, including celery, parsley, spinach, chamomile, green pepper, and eggplant, as well as oranges and red wine [173]. Apigenin is able to alleviate myeloperoxidase-mediated oxidative stress and repress ferroptosis in neuronal cells [174].
Resveratrol is a polyphenol that exists commonly in various vegetables and fruits, such as grapes. Resveratrol alleviates ferroptosis-induced myocardial ischemia/reperfusion injury, reduces TfR1 expression, and increases the expressions of FTH1 and GPX4 [175]. Resveratrol nanoparticles can repress erastin-induced ferroptosis in HT22 mouse hippocampal cells [176], and also inhibits acrolein-induced ferroptosis and insulin secretion disorder via the ER-stress-related PERK pathway in mouse pancreatic β cells [177]. Nobiletin, a critical active flavonoid in citrus fruits, was found to reduce ferroptosis-related renal injury, inflammation, and fibrosis in a unilateral ureteral obstruction mouse model [178]. Nobiletin triggers the ferroptosis of human skin melanoma cells via the GSK3β-mediated Keap1/Nrf2/HO-1 signaling pathway [179].
References
- Wu, S.; Zhu, C.; Tang, D.; Dou, Q.P.; Shen, J.; Chen, X. The role of ferroptosis in lung cancer. Biomark Res. 2021, 9, 82.
- Hu, K.; Li, K.; Lv, J.; Feng, J.; Chen, J.; Wu, H.; Cheng, F.; Jiang, W.; Wang, J.; Pei, H.; et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J. Clin. Invest. 2020, 130, 1752–1766.
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62.
- Gai, C.; Yu, M.; Li, Z.; Wang, Y.; Ding, D.; Zheng, J.; Lv, S.; Zhang, W.; Li, W. Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. J. Cell. Physiol. 2020, 235, 3329–3339.
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230.
- Yuan, B.; Liao, F.; Shi, Z.Z.; Ren, Y.; Deng, X.L.; Yang, T.T.; Li, D.Y.; Li, R.F.; Pu, D.D.; Wang, Y.J.; et al. Dihydroartemisinin Inhibits the Proliferation, Colony Formation and Induces Ferroptosis of Lung Cancer Cells by Inhibiting PRIM2/SLC7A11 Axis. Oncol. Targets Ther. 2020, 13, 10829–10840.
- Zhou, W.; Zhang, J.; Yan, M.; Wu, J.; Lian, S.; Sun, K.; Li, B.; Ma, J.; Xia, J.; Lian, C. Orlistat induces ferroptosis-like cell death of lung cancer cells. Front. Med. 2021, 15, 922–932.
- Wu, C.Y.; Yang, Y.H.; Lin, Y.S.; Chang, G.H.; Tsai, M.S.; Hsu, C.M.; Yeh, R.A.; Shu, L.H.; Cheng, Y.C.; Liu, H.T. Dihydroisotanshinone I induced ferroptosis and apoptosis of lung cancer cells. Biomed. Pharmacother. 2021, 139, 111585.
- Zhang, Q.; Yi, H.; Yao, H.; Lu, L.; He, G.; Wu, M.; Zheng, C.; Li, Y.; Chen, S.; Li, L.; et al. Artemisinin Derivatives Inhibit Non-small Cell Lung Cancer Cells Through Induction of ROS-dependent Apoptosis/Ferroptosis. J. Cancer 2021, 12, 4075–4085.
- Lu, X.; Kang, N.; Ling, X.; Pan, M.; Du, W.; Gao, S. MiR-27a-3p Promotes Non-Small Cell Lung Cancer Through SLC7A11-Mediated-Ferroptosis. Front. Oncol. 2021, 11, 759346.
- Wei, D.; Ke, Y.Q.; Duan, P.; Zhou, L.; Wang, C.Y.; Cao, P. MicroRNA-302a-3p induces ferroptosis of non-small cell lung cancer cells via targeting ferroportin. Free Radic. Res. 2021, 55, 821–830.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184.
- Yu, J.; Wang, J.Q. Research mechanisms of and pharmaceutical treatments for ferroptosis in liver diseases. Biochimie 2021, 180, 149–157.
- Louandre, C.; Marcq, I.; Bouhlal, H.; Lachaier, E.; Godin, C.; Saidak, Z.; François, C.; Chatelain, D.; Debuysscher, V.; Barbare, J.C.; et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015, 356, 971–977.
- Sun, X.; Niu, X.; Chen, R.; He, W.; Chen, D.; Kang, R.; Tang, D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology 2016, 64, 488–500.
- Bai, T.; Wang, S.; Zhao, Y.; Zhu, R.; Wang, W.; Sun, Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 491, 919–925.
- He, G.N.; Bao, N.R.; Wang, S.; Xi, M.; Zhang, T.H.; Chen, F.S. Ketamine Induces Ferroptosis of Liver Cancer Cells by Targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des. Devel. Ther. 2021, 15, 3965–3978.
- Wang, Z.; Li, M.; Liu, Y.; Qiao, Z.; Bai, T.; Yang, L.; Liu, B. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein response-induced upregulation of CHAC1 expression. Oncol. Rep. 2021, 46, 240.
- Bai, T.; Liang, R.; Zhu, R.; Wang, W.; Zhou, L.; Sun, Y. MicroRNA-214-3p enhances erastin-induced ferroptosis by targeting ATF4 in hepatoma cells. J. Cell. Physiol. 2020, 235, 5637–5648.
- Li, Z.J.; Dai, H.Q.; Huang, X.W.; Feng, J.; Deng, J.H.; Wang, Z.X.; Yang, X.M.; Liu, Y.J.; Wu, Y.; Chen, P.H.; et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol. Sin. 2021, 42, 301–310.
- Zhu, G.; Murshed, A.; Li, H.; Ma, J.; Zhen, N.; Ding, M.; Zhu, J.; Mao, S.; Tang, X.; Liu, L.; et al. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov. 2021, 7, 83.
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371.
- Wei, R.; Zhao, Y.; Wang, J.; Yang, X.; Li, S.; Wang, Y.; Yang, X.; Fei, J.; Hao, X.; Zhao, Y.; et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 2021, 17, 2703–2717.
- Zhang, L.; Liu, W.; Liu, F.; Wang, Q.; Song, M.; Yu, Q.; Tang, K.; Teng, T.; Wu, D.; Wang, X.; et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 1675613.
- Yang, C.; Zhang, Y.; Lin, S.; Liu, Y.; Li, W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging (Albany NY) 2021, 13, 13515–13534.
- Yang, J.; Mo, J.; Dai, J.; Ye, C.; Cen, W.; Zheng, X.; Jiang, L.; Ye, L. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021, 12, 1079.
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119.
- Xu, X.; Zhang, X.; Wei, C.; Zheng, D.; Lu, X.; Yang, Y.; Luo, A.; Zhang, K.; Duan, X.; Wang, Y. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur. J. Pharm. Sci. 2020, 152, 105450.
- Tian, X.; Li, S.; Ge, G. Apatinib Promotes Ferroptosis in Colorectal Cancer Cells by Targeting ELOVL6/ACSL4 Signaling. Cancer Manag. Res. 2021, 13, 1333–1342.
- Ye, S.; Xu, M.; Zhu, T.; Chen, J.; Shi, S.; Jiang, H.; Zheng, Q.; Liao, Q.; Ding, X.; Xi, Y. Cytoglobin promotes sensitivity to ferroptosis by regulating p53-YAP1 axis in colon cancer cells. J. Cell. Mol. Med. 2021, 25, 3300–3311.
- Sharma, P.; Shimura, T.; Banwait, J.K.; Goel, A. Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer. Carcinogenesis 2020, 41, 1385–1394.
- Yu, H.; Yang, C.; Jian, L.; Guo, S.; Chen, R.; Li, K.; Qu, F.; Tao, K.; Fu, Y.; Luo, F.; et al. Sulfasalazine-induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol. Rep. 2019, 42, 826–838.
- Ma, S.; Henson, E.S.; Chen, Y.; Gibson, S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307.
- Yang, J.; Zhou, Y.; Xie, S.; Wang, J.; Li, Z.; Chen, L.; Mao, M.; Chen, C.; Huang, A.; Chen, Y.; et al. Metformin induces Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 206.
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS ONE 2022, 17, e0261370.
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019, 110, 3173–3182.
- Wu, X.; Liu, C.; Li, Z.; Gai, C.; Ding, D.; Chen, W.; Hao, F.; Li, W. Regulation of GSK3β/Nrf2 signaling pathway modulated erastin-induced ferroptosis in breast cancer. Mol. Cell. Biochem. 2020, 473, 217–228.
- Song, X.; Wang, X.; Liu, Z.; Yu, Z. Role of GPX4-Mediated Ferroptosis in the Sensitivity of Triple Negative Breast Cancer Cells to Gefitinib. Front. Oncol. 2020, 10, 597434.
- Yao, X.; Xie, R.; Cao, Y.; Tang, J.; Men, Y.; Peng, H.; Yang, W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J. Nanobiotechnol. 2021, 19, 311.
- Hou, Y.; Cai, S.; Yu, S.; Lin, H. Metformin induces ferroptosis by targeting miR-324-3p/GPX4 axis in breast cancer. Acta Biochim. Biophys. Sin 2021, 53, 333–341.
- Sun, D.; Li, Y.C.; Zhang, X.Y. Lidocaine Promoted Ferroptosis by Targeting miR-382-5p /SLC7A11 Axis in Ovarian and Breast Cancer. Front. Pharmacol. 2021, 12, 681223.
- Li, H.; Liu, W.; Zhang, X.; Wu, F.; Sun, D.; Wang, Z. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Biochem. Biophys. Res. Commun. 2021, 585, 111–116.
- Tesfay, L.; Paul, B.T.; Konstorum, A.; Deng, Z.; Cox, A.O.; Lee, J.; Furdui, C.M.; Hegde, P.; Torti, F.M.; Torti, S.V. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019, 79, 5355–5366.
- Carbone, M.; Melino, G. Stearoyl CoA Desaturase Regulates Ferroptosis in Ovarian Cancer Offering New Therapeutic Perspectives. Cancer Res. 2019, 79, 5149–5150.
- Jin, Y.; Chen, L.; Li, L.; Huang, G.; Huang, H.; Tang, C. SNAI2 promotes the development of ovarian cancer through regulating ferroptosis. Bioengineered 2022, 13, 6451–6463.
- Mao, G.; Xin, D.; Wang, Q.; Lai, D. Sodium molybdate inhibits the growth of ovarian cancer cells via inducing both ferroptosis and apoptosis. Free Radic. Biol. Med. 2022, 182, 78–92.
- Hong, T.; Lei, G.; Chen, X.; Li, H.; Zhang, X.; Wu, N.; Zhao, Y.; Zhang, Y.; Wang, J. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021, 42, 101928.
- Zhang, Y.; Xia, M.; Zhou, Z.; Hu, X.; Wang, J.; Zhang, M.; Li, Y.; Sun, L.; Chen, F.; Yu, H. p53 Promoted Ferroptosis in Ovarian Cancer Cells Treated with Human Serum Incubated-Superparamagnetic Iron Oxides. Int. J. Nanomed. 2021, 16, 283–296.
- Li, H.W.; Liu, M.B.; Jiang, X.; Song, T.; Feng, S.X.; Wu, J.Y.; Deng, P.F.; Wang, X.Y. GALNT14 regulates ferroptosis and apoptosis of ovarian cancer through the EGFR/mTOR pathway. Future Oncol. 2022, 18, 149–161.
- Chan, D.W.; Yung, M.M.; Chan, Y.S.; Xuan, Y.; Yang, H.; Xu, D.; Zhan, J.B.; Chan, K.K.; Ng, T.B.; Ngan, H.Y. MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis. Pharmacol. Res. 2020, 161, 105157.
- Goral, V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac. J. Cancer Prev. 2015, 16, 5619–5624.
- Kremer, D.M.; Nelson, B.S.; Lin, L.; Yarosz, E.L.; Halbrook, C.J.; Kerk, S.A.; Sajjakulnukit, P.; Myers, A.; Thurston, G.; Hou, S.W.; et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat. Commun. 2021, 12, 4860.
- Eling, N.; Reuter, L.; Hazin, J.; Hamacher-Brady, A.; Brady, N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2015, 2, 517–532.
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89.
- Song, Z.; Xiang, X.; Li, J.; Deng, J.; Fang, Z.; Zhang, L.; Xiong, J. Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncol. Rep. 2020, 43, 516–524.
- Ye, Z.; Hu, Q.; Zhuo, Q.; Zhu, Y.; Fan, G.; Liu, M.; Sun, Q.; Zhang, Z.; Liu, W.; Xu, W.; et al. Abrogation of ARF6 promotes RSL3-induced ferroptosis and mitigates gemcitabine resistance in pancreatic cancer cells. Am. J. Cancer Res. 2020, 10, 1182–1193.
- Du, J.; Wang, X.; Li, Y.; Ren, X.; Zhou, Y.; Hu, W.; Zhou, C.; Jing, Q.; Yang, C.; Wang, L.; et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021, 12, 705.
- Wang, K.; Zhang, Z.; Wang, M.; Cao, X.; Qi, J.; Wang, D.; Gong, A.; Zhu, H. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des. Devel. Ther. 2019, 13, 2135–2144.
- Zhou, L.; Yang, C.; Zhong, W.; Wang, Q.; Zhang, D.; Zhang, J.; Xie, S.; Xu, M. Chrysin induces autophagy-dependent ferroptosis to increase chemosensitivity to gemcitabine by targeting CBR1 in pancreatic cancer cells. Biochem. Pharmacol. 2021, 193, 114813.
- Cui, W.; Zhang, J.; Wu, D.; Zhang, J.; Zhou, H.; Rong, Y.; Liu, F.; Wei, B.; Xu, X. Ponicidin suppresses pancreatic cancer growth by inducing ferroptosis: Insight gained by mass spectrometry-based metabolomics. Phytomedicine 2022, 98, 153943.
- Yamaguchi, Y.; Kasukabe, T.; Kumakura, S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022.
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste, M.E.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279.e25.
- Cui, Y.; Zhang, Y.; Zhao, X.; Shao, L.; Liu, G.; Sun, C.; Xu, R.; Zhang, Z. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav. Immun. 2021, 93, 312–321.
- Chen, J.; Yang, L.; Geng, L.; He, J.; Chen, L.; Sun, Q.; Zhao, J.; Wang, X. Inhibition of Acyl-CoA Synthetase Long-Chain Family Member 4 Facilitates Neurological Recovery After Stroke by Regulation Ferroptosis. Front. Cell. Neurosci. 2021, 15, 632354.
- Li, C.; Sun, G.; Chen, B.; Xu, L.; Ye, Y.; He, J.; Bao, Z.; Zhao, P.; Miao, Z.; Zhao, L.; et al. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol. Res. 2021, 174, 105933.
- Zille, M.; Oses-Prieto, J.A.; Savage, S.R.; Karuppagounder, S.S.; Chen, Y.; Kumar, A.; Morris, J.H.; Scheidt, K.A.; Burlingame, A.L.; Ratan, R.R. Hemin-Induced Death Models Hemorrhagic Stroke and Is a Variant of Classical Neuronal Ferroptosis. J. Neurosci. 2022, 42, 2065–2079.
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065.
- Duan, L.; Zhang, Y.; Yang, Y.; Su, S.; Zhou, L.; Lo, P.C.; Cai, J.; Qiao, Y.; Li, M.; Huang, S.; et al. Baicalin Inhibits Ferroptosis in Intracerebral Hemorrhage. Front. Pharmacol. 2021, 12, 629379.
- Tuo, Q.Z.; Lei, P.; Jackman, K.A.; Li, X.L.; Xiong, H.; Li, X.L.; Liuyang, Z.Y.; Roisman, L.; Zhang, S.T.; Ayton, S.; et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 2017, 22, 1520–1530.
- Xiao, Z.; Shen, D.; Lan, T.; Wei, C.; Wu, W.; Sun, Q.; Luo, Z.; Chen, W.; Zhang, Y.; Hu, L.; et al. Reduction of lactoferrin aggravates neuronal ferroptosis after intracerebral hemorrhagic stroke in hyperglycemic mice. Redox Biol. 2022, 50, 102256.
- Wang, F.; Li, W.L.; Shen, L.J.; Jiang, T.T.; Xia, J.J.; You, D.L.; Hu, S.Y.; Wang, L.; Wu, X. Crocin Alleviates Intracerebral Hemorrhage-Induced Neuronal Ferroptosis by Facilitating Nrf2 Nuclear Translocation. Neurotox. Res. 2022, 40, 596–604.
- Peng, C.; Fu, X.; Wang, K.; Chen, L.; Luo, B.; Huang, N.; Luo, Y.; Chen, W. Dauricine alleviated secondary brain injury after intracerebral hemorrhage by upregulating GPX4 expression and inhibiting ferroptosis of nerve cells. Eur. J. Pharmacol. 2022, 914, 174461.
- Zhang, H.; Wen, M.; Chen, J.; Yao, C.; Lin, X.; Lin, Z.; Ru, J.; Zhuge, Q.; Yang, S. Pyridoxal Isonicotinoyl Hydrazone Improves Neurological Recovery by Attenuating Ferroptosis and Inflammation in Cerebral Hemorrhagic Mice. Biomed Res. Int. 2021, 2021, 9916328.
- Xie, B.S.; Wang, Y.Q.; Lin, Y.; Mao, Q.; Feng, J.F.; Gao, G.Y.; Jiang, J.Y. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 2019, 25, 465–475.
- Kenny, E.M.; Fidan, E.; Yang, Q.; Anthonymuthu, T.S.; New, L.A.; Meyer, E.A.; Wang, H.; Kochanek, P.M.; Dixon, C.E.; Kagan, V.E.; et al. Ferroptosis Contributes to Neuronal Death and Functional Outcome After Traumatic Brain Injury. Crit. Care Med. 2019, 47, 410–418.
- Rui, T.; Wang, H.; Li, Q.; Cheng, Y.; Gao, Y.; Fang, X.; Ma, X.; Chen, G.; Gao, C.; Gu, Z.; et al. Deletion of ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis. J. Pineal Res. 2021, 70, e12704.
- Bao, Z.; Liu, Y.; Chen, B.; Miao, Z.; Tu, Y.; Li, C.; Chao, H.; Ye, Y.; Xu, X.; Sun, G.; et al. Prokineticin-2 prevents neuronal cell deaths in a model of traumatic brain injury. Nat. Commun. 2021, 12, 4220.
- Huang, L.; He, S.; Cai, Q.; Li, F.; Wang, S.; Tao, K.; Xi, Y.; Qin, H.; Gao, G.; Feng, D. Polydatin alleviates traumatic brain injury: Role of inhibiting ferroptosis. Biochem. Biophys. Res. Commun. 2021, 556, 149–155.
- Xiao, X.; Jiang, Y.; Liang, W.; Wang, Y.; Cao, S.; Yan, H.; Gao, L.; Zhang, L. miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol. Brain 2019, 12, 78.
- Chen, X.; Gao, C.; Yan, Y.; Cheng, Z.; Chen, G.; Rui, T.; Luo, C.; Gao, Y.; Wang, T.; Chen, X.; et al. Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Exp. Neurol. 2021, 342, 113762.
- Cheng, Y.; Qu, W.; Li, J.; Jia, B.; Song, Y.; Wang, L.; Rui, T.; Li, Q.; Luo, C. Ferristatin II, an Iron Uptake Inhibitor, Exerts Neuroprotection against Traumatic Brain Injury via Suppressing Ferroptosis. ACS Chem. Neurosci. 2022, 13, 664–675.
- Liu, H.; He, S.; Wang, J.; Li, C.; Liao, Y.; Zou, Q.; Chen, R. Tetrandrine Ameliorates Traumatic Brain Injury by Regulating Autophagy to Reduce Ferroptosis. Neurochem. Res. 2022, 47, 1574–1587.
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The Application of Ferroptosis in Diseases. Pharmacol. Res. 2020, 159, 104919.
- Bao, W.D.; Pang, P.; Zhou, X.T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562.
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947.
- Zhu, Z.Y.; Liu, Y.D.; Gong, Y.; Jin, W.; Topchiy, E.; Turdi, S.; Gao, Y.F.; Culver, B.; Wang, S.Y.; Ge, W.; et al. Mitochondrial aldehyde dehydrogenase (ALDH2) rescues cardiac contractile dysfunction in an APP/PS1 murine model of Alzheimer’s disease via inhibition of ACSL4-dependent ferroptosis. Acta Pharmacol. Sin. 2022, 43, 39–49.
- Gao, Y.; Li, J.; Wu, Q.; Wang, S.; Yang, S.; Li, X.; Chen, N.; Li, L.; Zhang, L. Tetrahydroxy stilbene glycoside ameliorates Alzheimer’s disease in APP/PS1 mice via glutathione peroxidase related ferroptosis. Int. Immunopharmacol. 2021, 99, 108002.
- Li, L.; Li, W.J.; Zheng, X.R.; Liu, Q.L.; Du, Q.; Lai, Y.J.; Liu, S.Q. Eriodictyol ameliorates cognitive dysfunction in APP/PS1 mice by inhibiting ferroptosis via vitamin D receptor-mediated Nrf2 activation. Mol. Med. 2022, 28, 11.
- Wang, C.; Chen, S.; Guo, H.; Jiang, H.; Liu, H.; Fu, H.; Wang, D. Forsythoside A Mitigates Alzheimer’s-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int. J. Biol. Sci. 2022, 18, 2075–2090.
- Tian, Y.; Lu, J.; Hao, X.; Li, H.; Zhang, G.; Liu, X.; Li, X.; Zhao, C.; Kuang, W.; Chen, D.; et al. FTH1 Inhibits Ferroptosis Through Ferritinophagy in the 6-OHDA Model of Parkinson’s Disease. Neurotherapeutics 2020, 17, 1796–1812.
- Sun, Y.; He, L.; Wang, T.; Hua, W.; Qin, H.; Wang, J.; Wang, L.; Gu, W.; Li, T.; Li, N.; et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Mol. Neurobiol. 2020, 57, 4628–4641.
- Bai, L.; Yan, F.; Deng, R.; Gu, R.; Zhang, X.; Bai, J. Thioredoxin-1 Rescues MPP+/MPTP-Induced Ferroptosis by Increasing Glutathione Peroxidase 4. Mol. Neurobiol. 2021, 58, 3187–3197.
- Si, W.; Huang, Z.; Li, X.; Zhao, L.; Ji, Y.; Li, H.; Liu, X.; Ye, S.; Chen, D.; Liu, H.; et al. Super-enhancer-driven Sorting Nexin 5 expression promotes dopaminergic neuronal ferroptosis in Parkinson’s disease models. Biochem. Biophys. Res. Commun. 2021, 567, 35–41.
- Li, X.; Si, W.; Li, Z.; Tian, Y.; Liu, X.; Ye, S.; Huang, Z.; Ji, Y.; Zhao, C.; Hao, X.; et al. miR-335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson’s disease. Int. J. Mol. Med. 2021, 47, 61.
- Huang, Z.; Si, W.; Li, X.; Ye, S.; Liu, X.; Ji, Y.; Hao, X.; Chen, D.; Zhu, M. Moxibustion Protects Dopaminergic Neurons in Parkinson’s Disease through Antiferroptosis. Evid. Based Complement. Altern. Med. 2021, 2021, 6668249.
- Lu, J.; Liu, X.; Tian, Y.; Li, H.; Ren, Z.; Liang, S.; Zhang, G.; Zhao, C.; Li, X.; Wang, T.; et al. Moxibustion Exerts a Neuroprotective Effect through Antiferroptosis in Parkinson’s Disease. Evid. Based Complement. Altern. Med. 2019, 2019, 2735492.
- Zuo, Y.; Xie, J.; Li, X.; Li, Y.; Thirupathi, A.; Zhang, J.; Yu, P.; Gao, G.; Chang, Y.; Shi, Z. Ferritinophagy-Mediated Ferroptosis Involved in Paraquat-Induced Neurotoxicity of Dopaminergic Neurons: Implication for Neurotoxicity in PD. Oxid. Med. Cell. Longev. 2021, 2021, 9961628.
- Mi, Y.; Gao, X.; Xu, H.; Cui, Y.; Zhang, Y.; Gou, X. The Emerging Roles of Ferroptosis in Huntington’s Disease. Neuromolecular Med. 2019, 21, 110–119.
- Tan, Q.; Fang, Y.; Gu, Q. Mechanisms of Modulation of Ferroptosis and Its Role in Central Nervous System Diseases. Front. Pharmacol. 2021, 12, 657033.
- Skouta, R.; Dixon, S.J.; Wang, J.; Dunn, D.E.; Orman, M.; Shimada, K.; Rosenberg, P.A.; Lo, D.C.; Weinberg, J.M.; Linkermann, A.; et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014, 136, 4551–4556.
- Chen, J.; Marks, E.; Lai, B.; Zhang, Z.; Duce, J.A.; Lam, L.Q.; Volitakis, I.; Bush, A.I.; Hersch, S.; Fox, J.H. Iron accumulates in Huntington’s disease neurons: Protection by deferoxamine. PLoS ONE 2013, 8, e77023.
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501.
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, e132747.
- Bai, Y.T.; Chang, R.; Wang, H.; Xiao, F.J.; Ge, R.L.; Wang, L.S. ENPP2 protects cardiomyocytes from erastin-induced ferroptosis. Biochem. Biophys. Res. Commun. 2018, 499, 44–51.
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102.
- Baba, Y.; Higa, J.K.; Shimada, B.K.; Horiuchi, K.M.; Suhara, T.; Kobayashi, M.; Woo, J.D.; Aoyagi, H.; Marh, K.S.; Kitaoka, H.; et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H659–H668.
- Song, Y.; Wang, B.; Zhu, X.; Hu, J.; Sun, J.; Xuan, J.; Ge, Z. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 2021, 37, 51–64.
- Park, T.J.; Park, J.H.; Lee, G.S.; Lee, J.Y.; Shin, J.H.; Kim, M.W.; Kim, Y.S.; Kim, J.Y.; Oh, K.J.; Han, B.S.; et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019, 10, 835.
- Hou, K.; Shen, J.; Yan, J.; Zhai, C.; Zhang, J.; Pan, J.A.; Zhang, Y.; Jiang, Y.; Wang, Y.; Lin, R.Z.; et al. Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine 2021, 69, 103456.
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954.
- Hao, L.; Mi, J.; Song, L.; Guo, Y.; Li, Y.; Yin, Y.; Zhang, C. SLC40A1 Mediates Ferroptosis and Cognitive Dysfunction in Type 1 Diabetes. Neuroscience 2021, 463, 216–226.
- Han, D.; Jiang, L.; Gu, X.; Huang, S.; Pang, J.; Wu, Y.; Yin, J.; Wang, J. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J. Cell. Physiol. 2020, 235, 8839–8851.
- Ma, H.; Wang, X.; Zhang, W.; Li, H.; Zhao, W.; Sun, J.; Yang, M. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid. Med. Cell. Longev. 2020, 2020, 9067610.
- Li, S.; Zheng, L.; Zhang, J.; Liu, X.; Wu, Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic. Biol. Med. 2021, 162, 435–449.
- Feng, X.; Wang, S.; Sun, Z.; Dong, H.; Yu, H.; Huang, M.; Gao, X. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice. Front. Endocrinol 2021, 12, 626390.
- Meng, Z.; Liang, H.; Zhao, J.; Gao, J.; Liu, C.; Ma, X.; Liu, J.; Liang, B.; Jiao, X.; Cao, J.; et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci. 2021, 284, 119935.
- Zang, H.; Wu, W.; Qi, L.; Tan, W.; Nagarkatti, P.; Nagarkatti, M.; Wang, X.; Cui, T. Autophagy Inhibition Enables Nrf2 to Exaggerate the Progression of Diabetic Cardiomyopathy in Mice. Diabetes 2020, 69, 2720–2734.
- Abdul, Y.; Li, W.; Ward, R.; Abdelsaid, M.; Hafez, S.; Dong, G.; Jamil, S.; Wolf, V.; Johnson, M.H.; Fagan, S.C.; et al. Deferoxamine Treatment Prevents Post-Stroke Vasoregression and Neurovascular Unit Remodeling Leading to Improved Functional Outcomes in Type 2 Male Diabetic Rats: Role of Endothelial Ferroptosis. Transl. Stroke Res. 2021, 12, 615–630.
- Tang, W.; Li, Y.; He, S.; Jiang, T.; Wang, N.; Du, M.; Cheng, B.; Gao, W.; Li, Y.; Wang, Q. Caveolin-1 alleviates diabetes-associated cognitive dysfunction through modulating neuronal ferroptosis-mediated mitochondrial homeostasis. Antioxid. Redox Signal. 2022.
- Chen, S.; Zhu, J.Y.; Zang, X.; Zhai, Y.Z. The Emerging Role of Ferroptosis in Liver Diseases. Front. Cell Dev. Biol. 2021, 9, 801365.
- Zhou, Z.; Ye, T.J.; DeCaro, E.; Buehler, B.; Stahl, Z.; Bonavita, G.; Daniels, M.; You, M. Intestinal SIRT1 Deficiency Protects Mice from Ethanol-Induced Liver Injury by Mitigating Ferroptosis. Am. J. Pathol. 2020, 190, 82–92.
- Yang, Y.; Chen, J.; Gao, Q.; Shan, X.; Wang, J.; Lv, Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology 2020, 445, 152599.
- Li, X.; Wang, T.X.; Huang, X.; Li, Y.; Sun, T.; Zang, S.; Guan, K.L.; Xiong, Y.; Liu, J.; Yuan, H.X. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 2020, 40, 1378–1394.
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020, 11, 144.
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739.
- Kong, Z.; Liu, R.; Cheng, Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 2019, 109, 2043–2053.
- Wu, A.; Feng, B.; Yu, J.; Yan, L.; Che, L.; Zhuo, Y.; Luo, Y.; Yu, B.; Wu, D.; Chen, D. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 2021, 46, 102131.
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411.
- Wang, L.; Zhang, Z.; Li, M.; Wang, F.; Jia, Y.; Zhang, F.; Shao, J.; Chen, A.; Zheng, S. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. Iubmb Life 2019, 71, 45–56.
- Yuan, S.; Wei, C.; Liu, G.; Zhang, L.; Li, J.; Li, L.; Cai, S.; Fang, L. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022, 55, e13158.
- Zhang, Z.; Yao, Z.; Wang, L.; Ding, H.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy 2018, 14, 2083–2103.
- Zhang, Z.; Guo, M.; Li, Y.; Shen, M.; Kong, D.; Shao, J.; Ding, H.; Tan, S.; Chen, A.; Zhang, F.; et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 2020, 16, 1482–1505.
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243.
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199.
- Friedmann, A.J.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191.
- Ma, D.; Li, C.; Jiang, P.; Jiang, Y.; Wang, J.; Zhang, D. Inhibition of Ferroptosis Attenuates Acute Kidney Injury in Rats with Severe Acute Pancreatitis. Dig. Dis. Sci. 2021, 66, 483–492.
- Ding, C.; Ding, X.; Zheng, J.; Wang, B.; Li, Y.; Xiang, H.; Dou, M.; Qiao, Y.; Tian, P.; Xue, W. miR-182-5p and miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell Death Dis. 2020, 11, 929.
- Zhao, Z.; Wu, J.; Xu, H.; Zhou, C.; Han, B.; Zhu, H.; Hu, Z.; Ma, Z.; Ming, Z.; Yao, Y.; et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis. 2020, 11, 629.
- Guo, L.; Zhang, T.; Wang, F.; Chen, X.; Xu, H.; Zhou, C.; Chen, M.; Yu, F.; Wang, S.; Yang, D.; et al. Targeted inhibition of Rev-erb-α/β limits ferroptosis to ameliorate folic acid-induced acute kidney injury. Br. J. Pharmacol. 2021, 178, 328–345.
- Yang, Y.; Cai, F.; Zhou, N.; Liu, S.; Wang, P.; Zhang, S.; Zhang, Y.; Zhang, A.; Jia, Z.; Huang, S. Dimethyl fumarate prevents ferroptosis to attenuate acute kidney injury by acting on NRF2. Clin. Transl. Med. 2021, 11, e382.
- Hu, Z.; Zhang, H.; Yi, B.; Yang, S.; Liu, J.; Hu, J.; Wang, J.; Cao, K.; Zhang, W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020, 11, 73.
- Chen, C.; Wang, D.; Yu, Y.; Zhao, T.; Min, N.; Wu, Y.; Kang, L.; Zhao, Y.; Du, L.; Zhang, M.; et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021, 12, 65.
- Yang, L.; Guo, J.; Yu, N.; Liu, Y.; Song, H.; Niu, J.; Gu, Y. Tocilizumab mimotope alleviates kidney injury and fibrosis by inhibiting IL-6 signaling and ferroptosis in UUO model. Life Sci. 2020, 261, 118487.
- Wang, Y.; Zhang, M.; Bi, R.; Su, Y.; Quan, F.; Lin, Y.; Yue, C.; Cui, X.; Zhao, Q.; Liu, S.; et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022, 51, 102262.
- Liu, P.; Feng, Y.; Li, H.; Chen, X.; Wang, G.; Xu, S.; Li, Y.; Zhao, L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020, 25, 10.
- Dong, H.; Qiang, Z.; Chai, D.; Peng, J.; Xia, Y.; Hu, R.; Jiang, H. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY) 2020, 12, 12943–12959.
- Li, Y.; Cao, Y.; Xiao, J.; Shang, J.; Tan, Q.; Ping, F.; Huang, W.; Wu, F.; Zhang, H.; Zhang, X. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020, 27, 2635–2650.
- Li, J.; Lu, K.; Sun, F.; Tan, S.; Zhang, X.; Sheng, W.; Hao, W.; Liu, M.; Lv, W.; Han, W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021, 19, 96.
- Fan, R.; Sui, J.; Dong, X.; Jing, B.; Gao, Z. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free Radic. Biol. Med. 2021, 173, 29–40.
- Qiu, Y.B.; Wan, B.B.; Liu, G.; Wu, Y.X.; Chen, D.; Lu, M.D.; Chen, J.L.; Yu, R.Q.; Chen, D.Z.; Pang, Q.F. Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis. Respir. Res. 2020, 21, 232.
- Qiang, Z.; Dong, H.; Xia, Y.; Chai, D.; Hu, R.; Jiang, H. Nrf2 and STAT3 Alleviates Ferroptosis-Mediated IIR-ALI by Regulating SLC7A11. Oxid. Med. Cell. Longev. 2020, 2020, 5146982.
- Fan, G.; Zhu, T.; Min, X.; Xiong, J. Melatonin protects against PM2.5-induced lung injury by inhibiting ferroptosis of lung epithelial cells in a Nrf2-dependent manner. Ecotoxicol. Environ. Saf. 2021, 223, 112588.
- Liu, X.; Wang, L.; Xing, Q.; Li, K.; Si, J.; Ma, X.; Mao, L. Sevoflurane inhibits ferroptosis: A new mechanism to explain its protective role against lipopolysaccharide-induced acute lung injury. Life Sci. 2021, 275, 119391.
- Li, J.; Deng, S.H.; Li, J.; Li, L.; Zhang, F.; Zou, Y.; Wu, D.M.; Xu, Y. Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell. Mol. Biol. Lett. 2022, 27, 29.
- Li, J.; Li, M.; Li, L.; Ma, J.; Yao, C.; Yao, S. Hydrogen sulfide attenuates ferroptosis and stimulates autophagy by blocking mTOR signaling in sepsis-induced acute lung injury. Mol. Immunol. 2022, 141, 318–327.
- Xu, Y.; Li, X.; Cheng, Y.; Yang, M.; Wang, R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J. 2020, 34, 16262–16275.
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020, 11, 86.
- Chen, Y.; Zhang, P.; Chen, W.; Chen, G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol. Lett. 2020, 225, 9–15.
- Wang, S.; Liu, W.; Wang, J.; Bai, X. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci. 2020, 259, 118356.
- Xu, J.; Liu, S.; Cui, Z.; Wang, X.; Ning, T.; Wang, T.; Zhang, N.; Xie, S.; Min, L.; Zhang, S.; et al. Ferrostatin-1 alleviated TNBS induced colitis via the inhibition of ferroptosis. Biochem. Biophys. Res. Commun. 2021, 573, 48–54.
- Dong, S.; Lu, Y.; Peng, G.; Li, J.; Li, W.; Li, M.; Wang, H.; Liu, L.; Zhao, Q. Furin inhibits epithelial cell injury and alleviates experimental colitis by activating the Nrf2-Gpx4 signaling pathway. Dig. Liver Dis. 2021, 53, 1276–1285.
- Chen, Y.; Wang, J.; Li, J.; Zhu, J.; Wang, R.; Xi, Q.; Wu, H.; Shi, T.; Chen, W. Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur. J. Pharmacol. 2021, 911, 174518.
- Mayr, L.; Grabherr, F.; Schwärzler, J.; Reitmeier, I.; Sommer, F.; Gehmacher, T.; Niederreiter, L.; He, G.W.; Ruder, B.; Kunz, K.; et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat. Commun. 2020, 11, 1775.
- Li, X.; Zeng, J.; Liu, Y.; Liang, M.; Liu, Q.; Li, Z.; Zhao, X.; Chen, D. Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells. Antioxidants 2020, 9, 205.
- Wang, Z.X.; Ma, J.; Li, X.Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.M.; Lu, G.D.; Zhou, J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148.
- Liu, X.; Ma, Y.; Luo, L.; Zong, D.; Li, H.; Zeng, Z.; Cui, Y.; Meng, W.; Chen, Y. Dihydroquercetin suppresses cigarette smoke induced ferroptosis in the pathogenesis of chronic obstructive pulmonary disease by activating Nrf2-mediated pathway. Phytomedicine 2022, 96, 153894.
- Tang, H.M.; Cheung, P. Gallic Acid Triggers Iron-Dependent Cell Death with Apoptotic, Ferroptotic, and Necroptotic Features. Toxins 2019, 11, 492.
- Khorsandi, K.; Kianmehr, Z.; Hosseinmardi, Z.; Hosseinzadeh, R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020, 20, 18.
- Guerrero-Hue, M.; García-Caballero, C.; Palomino-Antolín, A.; Rubio-Navarro, A.; Vázquez-Carballo, C.; Herencia, C.; Martín-Sanchez, D.; Farré-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019, 33, 8961–8975.
- Xie, L.W.; Cai, S.; Zhao, T.S.; Li, M.; Tian, Y. Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic. Biol. Med. 2020, 161, 175–186.
- He, H.; Wang, L.; Qiao, Y.; Yang, B.; Yin, D.; He, M. Epigallocatechin-3-gallate pretreatment alleviates doxorubicin-induced ferroptosis and cardiotoxicity by upregulating AMPKα2 and activating adaptive autophagy. Redox Biol. 2021, 48, 102185.
- Lesjak, M.; Simin, N.; Srai, S. Can Polyphenols Inhibit Ferroptosis? Antioxidants 2022, 11, 150.
- Shao, C.; Yuan, J.; Liu, Y.; Qin, Y.; Wang, X.; Gu, J.; Chen, G.; Zhang, B.; Liu, H.K.; Zhao, J.; et al. Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc. Natl. Acad Sci. USA 2020, 117, 10155–10164.
- Li, T.; Tan, Y.; Ouyang, S.; He, J.; Liu, L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 2022, 808, 145968.
- Mo, Y.; Duan, L.; Yang, Y.; Liu, W.; Zhang, Y.; Zhou, L.; Su, S.; Lo, P.C.; Cai, J.; Gao, L.; et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale 2021, 13, 3827–3840.
- Zhang, X.; Jiang, L.; Chen, H.; Wei, S.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress- related PERK pathway in MIN6 cells. Toxicology 2022, 465, 153048.
- Lo, Y.H.; Yang, S.F.; Cheng, C.C.; Hsu, K.C.; Chen, Y.S.; Chen, Y.Y.; Wang, C.W.; Guan, S.S.; Wu, C.T. Nobiletin Alleviates Ferroptosis-Associated Renal Injury, Inflammation, and Fibrosis in a Unilateral Ureteral Obstruction Mouse Model. Biomedicines 2022, 10, 595.
- Feng, S.; Zhou, Y.; Huang, H.; Lin, Y.; Zeng, Y.; Han, S.; Huang, K.; Liu, Q.; Zhu, W.; Yuan, Z.; et al. Nobiletin Induces Ferroptosis in Human Skin Melanoma Cells Through the GSK3β-Mediated Keap1/Nrf2/HO-1 Signalling Pathway. Front. Genet. 2022, 13, 865073.
More
