Several eye-catching techniques have been developed to implement high-quality optical thin films for light-guiding applications [29]. Thin films are the foundation for innovative technologies in various areas, including optical devices, environmental applications, telecommunications devices, and energy storage devices [30]. The morphology and reliability of thin films are critical issues in all applications. Deposition techniques have a major influence on thin-film morphology. Physical and chemical deposition methods can be used to deposit high-quality thin films. A thin film is a thin layer of material with a thickness ranging from a few nm to a few μm. Thin films, like all materials, are classified as amorphous or polycrystalline based on the preparation conditions and the quality of the target material. Glass WGs display highly attractive properties due to the straightforward technology, the low propagation losses, and the flexible index matching to glass fibers. It is highly desirable to have low-loss glasses, reliable and enabling low-cost WG fabrication procedures. An overall requirement is that manufacturing technologies are proficient in high yield, and have guaranteed duplicability within the quantified tolerances, and fundamentally low operating costs.
- sol-gel dip-coating method
- chemical vapor deposition
- silica-titania waveguide platform
- ion exchange
1. Thin-Film Deposition Techniques
1.1. Physical Vapor Deposition Techniques (PVD)
Thermal evaporation method [3][41], electron beam evaporation [4][42], pulsed laser evaporation [5][43], molecular beam epitaxy [6][44], ion plating [7][45] and activated reactive evaporation [8][46] are all examples of PVD methods. This deposition process aims to move atoms from a source to a substrate, where film-forming and growth can happen independently. However, there are some disadvantages, such as the need for a tightly controlled vacuum environment and expensive instrumentation. PVD is a vaporization coating technique that requires an atomic-level material transfer. It is a vacuum-based process in which vaporized material from a source is transferred through a vacuum or low-pressure gas atmosphere to a substrate, where it condenses.- A.
-
Vacuum
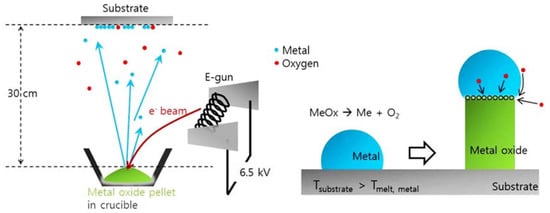 Figure 12. Diagrams of the E-beam evaporation model for the growth of metal oxide nanowire and growth process by vapor-liquid-solid [10][48].Another physical deposition method for the thin-film-coating system is pulsed-laser deposition (PLD), in which the laser beam is used to ablate the target material for depositing thin films in a vacuum chamber [18][56]. Different types of laser sources are used to ablate the target, such as Nd-YAG laser, KrF (248 nm), and XeCl (308 nm). When a laser beam hits a target material, it creates a plume that can be deposited on several substrates. The plume can be composed of ionized species as well as neutral and ground-state atoms. To acquire metal oxide thin films, oxygen is used in the process. The quality of thin film deposited by the PLD method is determined by various factors, including the wavelength of the laser, energy, atmospheric gas pressure, pulse size, and the target-to-substrate distance [19][57]. As illustrated recently, PLD has been used to deposit an extremely diverse variety of materials. The most important use of PLD in the past has been demonstrated in high-temperature superconducting thin films. The experiment revealing that PLD could be used to deposit YBa2Cu3O7−x (YBCO) films with zero resistivity at nearly 85 K triggered intensive research on the high-temperature superconductivity over the last decade, as well as on PLD in general [20][58]. For detailed knowledge about the PLD method, please consult [5][43].
Figure 12. Diagrams of the E-beam evaporation model for the growth of metal oxide nanowire and growth process by vapor-liquid-solid [10][48].Another physical deposition method for the thin-film-coating system is pulsed-laser deposition (PLD), in which the laser beam is used to ablate the target material for depositing thin films in a vacuum chamber [18][56]. Different types of laser sources are used to ablate the target, such as Nd-YAG laser, KrF (248 nm), and XeCl (308 nm). When a laser beam hits a target material, it creates a plume that can be deposited on several substrates. The plume can be composed of ionized species as well as neutral and ground-state atoms. To acquire metal oxide thin films, oxygen is used in the process. The quality of thin film deposited by the PLD method is determined by various factors, including the wavelength of the laser, energy, atmospheric gas pressure, pulse size, and the target-to-substrate distance [19][57]. As illustrated recently, PLD has been used to deposit an extremely diverse variety of materials. The most important use of PLD in the past has been demonstrated in high-temperature superconducting thin films. The experiment revealing that PLD could be used to deposit YBa2Cu3O7−x (YBCO) films with zero resistivity at nearly 85 K triggered intensive research on the high-temperature superconductivity over the last decade, as well as on PLD in general [20][58]. For detailed knowledge about the PLD method, please consult [5][43].- B.
-
Sputtering techniquesSputtering is a physical vapor deposition procedure with a high film deposition rate and low-temperature structures, making it a good technique [21][22][67,68]. It is fast and inexpensive to create thin films of alloys, metals, nitrides, carbides, and oxides [23][24][25][69,70,71]. The magnetron sputtering technique, which uses a magnetic field to support the process of depositing thin films onto a substrate, is the most common procedure for this technique [26][72]. Via the momentum transfer from the argon (Ar) ions, the particles (atoms and ions) are discharged. Electrons are confined to the magnetic field lines in magnetron sputtering. The target is bombarded with a gaseous plasma that keeps electrons and is then guided to grind down the material and expel them in the shape of neutral particles and a small portion of ions. An inactive gas such as Ar, or even an active gas, such as nitrogen, is widely used as sputter gas. The expelled particles would then settle on the substrate and form a thin layer of the target material.Magnetron sputtering has many benefits over other methods, including uniformity, smoothness, and strong adhesion deposition over a very substantial region. The ability to select substrate and target materials with high melting points and a high deposition rate enables simple manipulation of deposited layer thickness [27][73]. However, the reactive sputtering method has a variety of drawbacks, including target poisoning, low deposition rates, and arcing that produces imperfections in thin films [28][74]. In the sputtering techniques, however, several key factors are employed to adjust the thickness of the manufactured films. The integrated pulse energy, time of deposition, pressure in the chamber, plasma gas, target-to-substrate angle, and substrate temperature are all crucial elements in diminishing dopant redistribution and defect creation during high-temperature processing. There are several types of high-quality thin films, such as boron carbon nitride [22][68], aluminum oxide [29][75], gallium oxide [30][76], and others [31][77], deposited via the magnetron sputtering technique [21][67]. TFor readers interested in the history of the thin-film sputter deposition process can, we recommend consulting the reference [32][78].
1.2. Chemical Deposition Techniques
In chemical deposition techniques, materials to be deposited are permitted to respond to different chemicals, thereby permitting reactions to occur in a way that high-quality thin films are successfully deposited. Chemical deposition techniques involve gas-phase and liquid-phase deposition methods as discussed in the following section.- A.
-
Gas-phase
- B.
-
Liquid-phase deposition
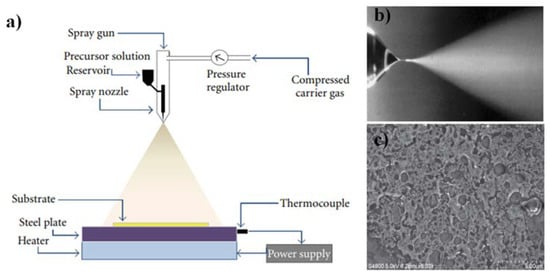 Figure 23. (a) The schematic of the spray pyrolysis setup [51][99], (b) Cone-jet spraying of methanol containing a small amount of HCL. Reprinted with permission from [62][110], (c) FESEM micrograph of SnO2 thin film [51][99].B.2. Sol-gel method The sol-gel method, which involves a suspension of colloidal particles, was invented at the dawn of chemistry. The groundbreaking work of Ebelmen (a French chemist) in the 1800s is credited with establishing a sol-gel synthesis of silicon tetra isoamyl oxide from silicon tetrachloride and isoamyl alcohol [63][111]. The latter study included the synthesis of boron amyloxide, boron ethoxide, and boron methoxide using isoamyl alcohol, ethanol, and methanol, respectively, with boron trichloride [63][111]. The sol-gel method is a wet chemical method for creating thin-film coatings. Its key benefits are the overall low cost of the procedure relative to more conventional processes such as CVD and PVD, as well as the ability to adapt the composition and properties of thin films to adjust the requirements of the anticipated application [64][112]. Thin-film coating methods must fulfill the requirements of complete control of film thickness to be successfully used in integrated optics. As a result, thickness management is critical for thin-film development processes in general, and sol-gel is no exception. It has been indicated that the final thickness is primarily determined by coating speed, angle of inclination, and sol concentration. Besides, sol viscosity, density, and liquid-vapor surface tension can also influence the final heat-treated thickness [65][113]. According to [66][114], the coating process must be carried out in a cleanroom environment to acquire sol-gel thin films of high optical quality. In [67][115], a three-step sol-gel process was established to make organic dye-doped thin films with customized porosity for applications in chemical sensing and optoelectronics. Moreover, sol-gel-derived ceramic films are also presented in [68][116]. More significant works on the sol-gel method can be found here [69][70][71][72][73][74][75][76][77][78][117,118,119,120,121,122,123,124,125,126]. To understand the popularity of the sol-gel and other traditional methods, scholarswe have plotted the number of research papers published on the sol-gel method, CVD, and RF-sputtering from the year 1990 to 2021, as shown in Figure 34. The data has been taken from the Scopus database, which is one of the authentic databases like Web of Science and Google Scholar. From 1990 to 2004, quite intensive research was conducted on CVD while the sol-gel method was still growing. After 2004, the sol-gel method gained more recognition for the deposition of high-quality thin films. The RF-sputtering method is also widely used in research but does not seem to be as prevalent as the other two methods.
Figure 23. (a) The schematic of the spray pyrolysis setup [51][99], (b) Cone-jet spraying of methanol containing a small amount of HCL. Reprinted with permission from [62][110], (c) FESEM micrograph of SnO2 thin film [51][99].B.2. Sol-gel method The sol-gel method, which involves a suspension of colloidal particles, was invented at the dawn of chemistry. The groundbreaking work of Ebelmen (a French chemist) in the 1800s is credited with establishing a sol-gel synthesis of silicon tetra isoamyl oxide from silicon tetrachloride and isoamyl alcohol [63][111]. The latter study included the synthesis of boron amyloxide, boron ethoxide, and boron methoxide using isoamyl alcohol, ethanol, and methanol, respectively, with boron trichloride [63][111]. The sol-gel method is a wet chemical method for creating thin-film coatings. Its key benefits are the overall low cost of the procedure relative to more conventional processes such as CVD and PVD, as well as the ability to adapt the composition and properties of thin films to adjust the requirements of the anticipated application [64][112]. Thin-film coating methods must fulfill the requirements of complete control of film thickness to be successfully used in integrated optics. As a result, thickness management is critical for thin-film development processes in general, and sol-gel is no exception. It has been indicated that the final thickness is primarily determined by coating speed, angle of inclination, and sol concentration. Besides, sol viscosity, density, and liquid-vapor surface tension can also influence the final heat-treated thickness [65][113]. According to [66][114], the coating process must be carried out in a cleanroom environment to acquire sol-gel thin films of high optical quality. In [67][115], a three-step sol-gel process was established to make organic dye-doped thin films with customized porosity for applications in chemical sensing and optoelectronics. Moreover, sol-gel-derived ceramic films are also presented in [68][116]. More significant works on the sol-gel method can be found here [69][70][71][72][73][74][75][76][77][78][117,118,119,120,121,122,123,124,125,126]. To understand the popularity of the sol-gel and other traditional methods, scholarswe have plotted the number of research papers published on the sol-gel method, CVD, and RF-sputtering from the year 1990 to 2021, as shown in Figure 34. The data has been taken from the Scopus database, which is one of the authentic databases like Web of Science and Google Scholar. From 1990 to 2004, quite intensive research was conducted on CVD while the sol-gel method was still growing. After 2004, the sol-gel method gained more recognition for the deposition of high-quality thin films. The RF-sputtering method is also widely used in research but does not seem to be as prevalent as the other two methods.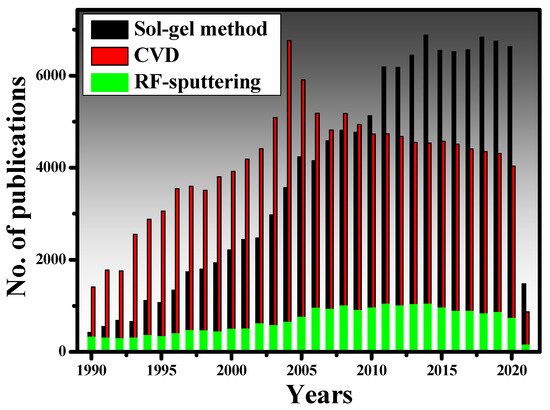 Figure 34. The number of publications related to CVD, RF-sputtering, and sol-gel method, indexed in the Scopus database. The keywords “sol-gel dip coating method”, “Chemical vapor deposition” and “RF-sputtering” were used during the search.
Figure 34. The number of publications related to CVD, RF-sputtering, and sol-gel method, indexed in the Scopus database. The keywords “sol-gel dip coating method”, “Chemical vapor deposition” and “RF-sputtering” were used during the search.2. Refractive Index Modification Methods
The refractive index (RI) of a material is a number that describes how the light will propagate through it. Light travels at different speeds in the materials having different RI, which can be changed by modifying the density of the material.
2.1. Ion Exchange Process
Ion exchange is a primeval method focused on replacing an ion existing in the glass (typically Na+) with another ion (e.g., Ag+, K+, and Li+) typically provided by a salt melt [79][127]. It dates to the first era as a technique for painting glass: it seems that Egyptians previously utilized it in the sixth century to embellish plates and vessels with a brownish-yellow color. The Moors used this method to dye the window glass of their palaces in Spain a few centuries later. In the 1960s, the solidification of the glass surface by ion-exchange transformed into a routine industrial procedure. Since the early 1970s, the appropriateness of ion exchange technology for the development of optical WGs in glass has been known as the groundbreaking works of Izawa and Nakagome [80][128] and Giallorenzi et al. [81][129] demonstrated techniques to benefit from the upsurge in the RI of integrated optics created by replacing the Na+ with another ion having higher electronic polarizability, for instance, silver (Ag).Glass is a well-known optical medium, and glass WGs offer several benefits, notably inexpensive material costs, compliance with optical fibers, low propagation loss and birefringence, and good stability and durability. Ion exchange as a fabrication method offers convenience and cost savings because it does not require complex production equipment. It supports batch processing making it adaptable to a wide range of applications. The ion exchange method has also been shown to be suitable for the industrial manufacture of WG components; however, unique fabrication criteria for modules that will be used in the field must be met. After more than 10 years into ion-exchanged WGs, interest has steadily evolved, and basic demonstrations of the practicability of single-mode devices and the process’ adaptability have laid the groundwork for the technology’s prospects, such as a serious commercial production possibility frontier. Findakly examined the state of ion-exchanged glass WG available technology [82][130].2.2. Ion Implantation
Ion implantation has shown to be an effective method for manufacturing optical WGs in different substrates [83][84][141,142]. In the instance of light ions being inserted, the physical density of the substrate is decreased by the damage instigated by nuclear collisions during implantation. As a result, the region with a RI lower than the substrate can function as an optical barrier that allows the light confinement in a thin layer between the surface of the substrate and the optical barrier. However, this optical confinement can be somewhat weak, and the alleged tunneling effect may occur, resulting in the energy leakage of the propagating light. To diminish light leakage to the substrate through the optical barrier, multiple-energy implants are frequently employed to extend the barrier width [85][143]. Heavy ions, on the other hand, may upsurge the physical density and polarizability of the substrate, which raises the RI of the implanted region bounded by the region with a lower RI, resulting in a typical optical WG. The exact precision of implantation depth and the number of doped ions employed are two main benefits of employing ion implantation. The implantation depth is proportional to the acceleration voltage, which is generally 10–100 keV, while the ion current may be used to measure the number of ions (called the dosage). The ion implantation process is complicated, and the readers who seek in-depth knowledge on this topic are referred to [86][144].2.3. Femtosecond-Laser Writing
Integrated optics can be more readily miniaturized and incorporated with micro-electronics resulting in accurate and exceedingly integrated systems, contrary to many optical fiber technologies. In 1996, femtosecond (fs) laser writing was first established and had been thoroughly investigated ever since [87][147]. Due to its ability to swiftly and flexibly direct the inscription of the complex structures with satisfactory accuracy, fs-laser WG writing in the glass is an encouraging method for integrated optics [88][148]. Photolithography and FIB micromachining, on the other hand, are slower. In addition, materials, including glasses [89][90][149,150], crystals [91][92][19,151], and polymers [93][94][152,153], WG structures can now be written on directly. Compared to the most popular techniques for the development of WG, fs-laser writing has the advantages of quick production, versatility in WG design, high three-dimensional accuracy, and easy integration of the resulting WG structures with optical fiber E-beam lithography and PECVD. It is interesting to develop integrated photonics WG sensors that do not involve any etching or complex fabrication procedures. For that reason, the WG should be written near the surface of the substrate. In most previous studies [95][96][97][154,155,156], the WGs were embedded inside the glass substrate via fs-laser writing. However, there are still a few reported studies on the laser-written WGs near the surface of the substrate [98][99][100][157,158,159]. This situation is mostly due to the ablation of glass that happens near the surface while focusing. Writing near-surface WGs is inspired by the desire to allow light to interact with the ambient medium to create integrated sensors in glass chips. Fast fabrication, flexibility in WG design, high spatial precision (i.e., limited by beam quality, wavelength, and polarization), and simple integration of the manufactured WGs with fiberized modules are all advantages of fs-laser direct writing over the most common methods, EBL and PECVD.
