The widespread application of metal-based nanoparticles (MNPs) has prompted great interest in nano-biosafety. Consequently, as more and more MNPs are released into the environment and eventually sink into the soil, plants, as an essential component of the ecosystem, are at greater risk of exposure and response to these MNPs. Therefore, to understand the potential impact of nanoparticles on the environment, their effects should be thoroughly investigated.
Arabidopsis
(
Arabidopsis thaliana
L.) is an ideal model plant for studying the impact of environmental stress on plants’ growth and development because the ways in which
Arabidopsis
adapt to these stresses resemble those of many plants, and therefore, conclusions obtained from these scientific studies have often been used as the universal reference for other plants.
- metal-based nanoparticles
- Arabidopsis thaliana
1. Introduction
2. Absorption and Transport of MNPs in Arabidopsis thaliana
Based on studies performed to assess the ecological safety of nanomaterials, it is generally believed that nanoparticles exposed to the surface of plants can be attached to tissue surfaces and hinder the transmission of water, nutrients and ion exchange. Some hydrophilic nanoparticles have been found to cross plants’ cell walls and accumulate between cell walls and cell membranes or between cell walls of adjacent cells, indicating a potential plasmatic exosomal transport mode for nanoparticles in plant tissues. Despite limited evidence showing that intact plant roots can absorb and translocate nanoparticles [18[18][19][20][21],19,20,21], there are still controversies surrounding this issue [22,23][22][23].2.1. Absorption and Transport of Monometallic Nanoparticles in Arabidopsis thaliana
Geisler-Lee et al. tested different sizes of Ag NPs (20, 40, 80 nm) in a hydroponic growth media using different microscopy methods to study the effects of Ag NPs’ toxicity in Arabidopsis root tips. They found that Ag NPs were absorbed and gradually accumulated in the root tips, from the marginal cells to the root cap, epidermis and columella, and then penetrated the initial part of the root meristem (Figure 1a). At low concentrations, smaller Ag NPs accumulated more than larger ones, while at high concentrations, the opposite occurred [24,25][24][25]. Ag NPs were first absorbed by underground tissues (primary root and lateral roots) and then transferred to aboveground parts (stems, leaves, flowers, etc.) where they tended to influence the growth and development of Arabidopsis thaliana. They appeared to accumulate in the plastid exosomes of root tissues while only a tiny fraction was transported to aboveground tissues. In the places they accumulated, i.e., on the surface of bare plant roots and leaves, they demonstrated a low internalization rate. It was found that the particle size of Ag NPs in plant tissues was larger than their initial diameter, suggesting that the internalized Ag NPs no longer existed as intact individual particles but rather appeared to aggregate and biotransform in the plants [26]. In contrast to observations made for Ag NPs, Au NPs (60 nm) tended to remain unaggregated after being absorbed by Arabidopsis roots. Yeonjong Koo et al. compared leaf acoustic signal distributions from Arabidopsis leaves exposed to media with high (2.4 × 1010 NP mL−2) or low (4.8 × 108 NP mL−2) GNP concentrations. The high GNP concentration increased the percentage of the leaf surface area, but regardless of concentration, nearly all the signals remained in the 90–200 mV amplitude range. A lack of high-amplitude signals suggests that GNPs did not aggregate in plants (Figure 1b) [27]. Thus, it seems that in addition to the changes in morphology and concentrations of monometallic nanomaterials that occur in Arabidopsis, other factors also affect the state of MNPs in plants.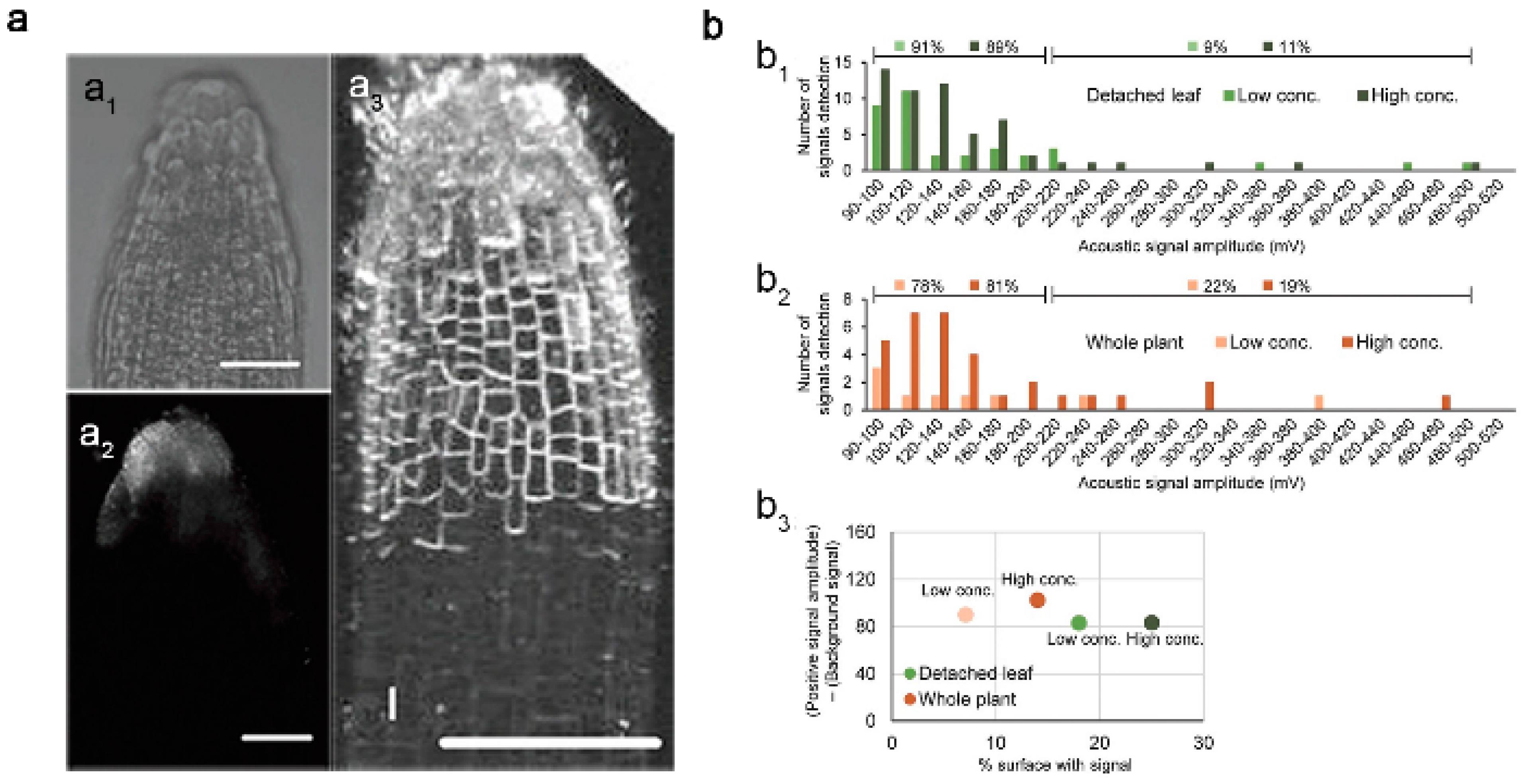
2.2. Absorption and Transport of Metal Oxide Nanoparticles in Arabidopsis thaliana
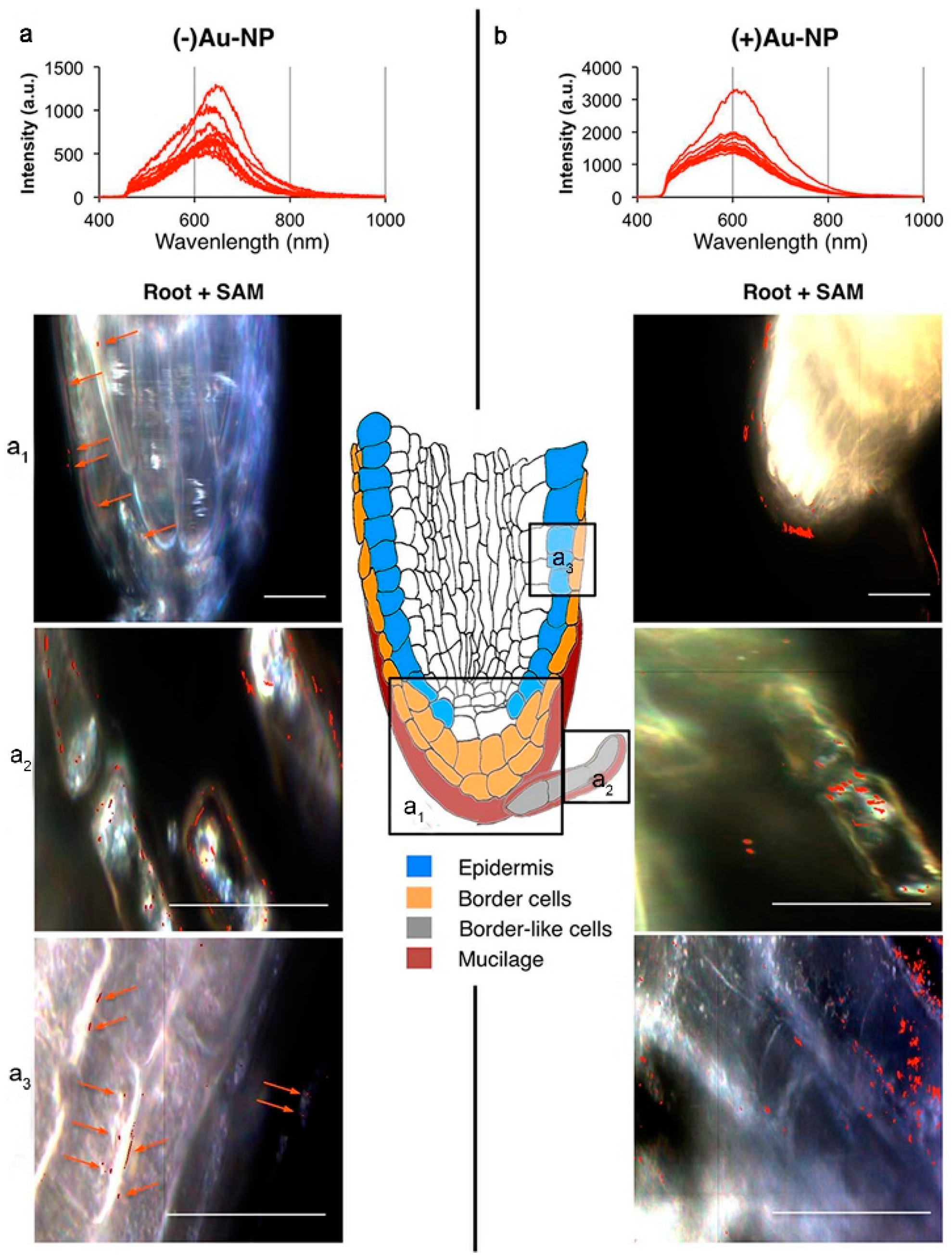
The use of zinc oxide nanoparticles (ZnO NPs) as Zn fertilizer has been shown to be effective for correcting Zn deficiency in soils [31]. However, it has also been shown that ZnO NPs may dissolve rapidly once they are released into the soil, releasing Zn ions, and may lead to a far higher concentration of Zn than expected [32]. In plants, Zn homeostasis is mediated through transporter proteins involved in the intracellular acquisition of Zn, mobilization and sequestration [33]. The Arabidopsis transporter proteins AtZIP4, AtZIP9 and AtZIP12 are involved in the acquisition of Zn from roots and subsequent mobilization to aerial tissues, while AtHMA3 and AtHMA4 mediate root-to-crown Zn transport [34,35][34][35]. Prakash et al. observed Arabidopsis seedlings after treatment with ZnO NPs under fluorescent labeling. They detected an intense green fluorescence in the primordial root tip region, primordial lateral root junctions and aboveground root junctions, but ZnO NPs treatment resulted in Zn accumulation only in the root apex and root–shoot junctions, whereas Zn ion treatment caused a root-to-shoot uptake and translocation of the element (Figure 3) [36].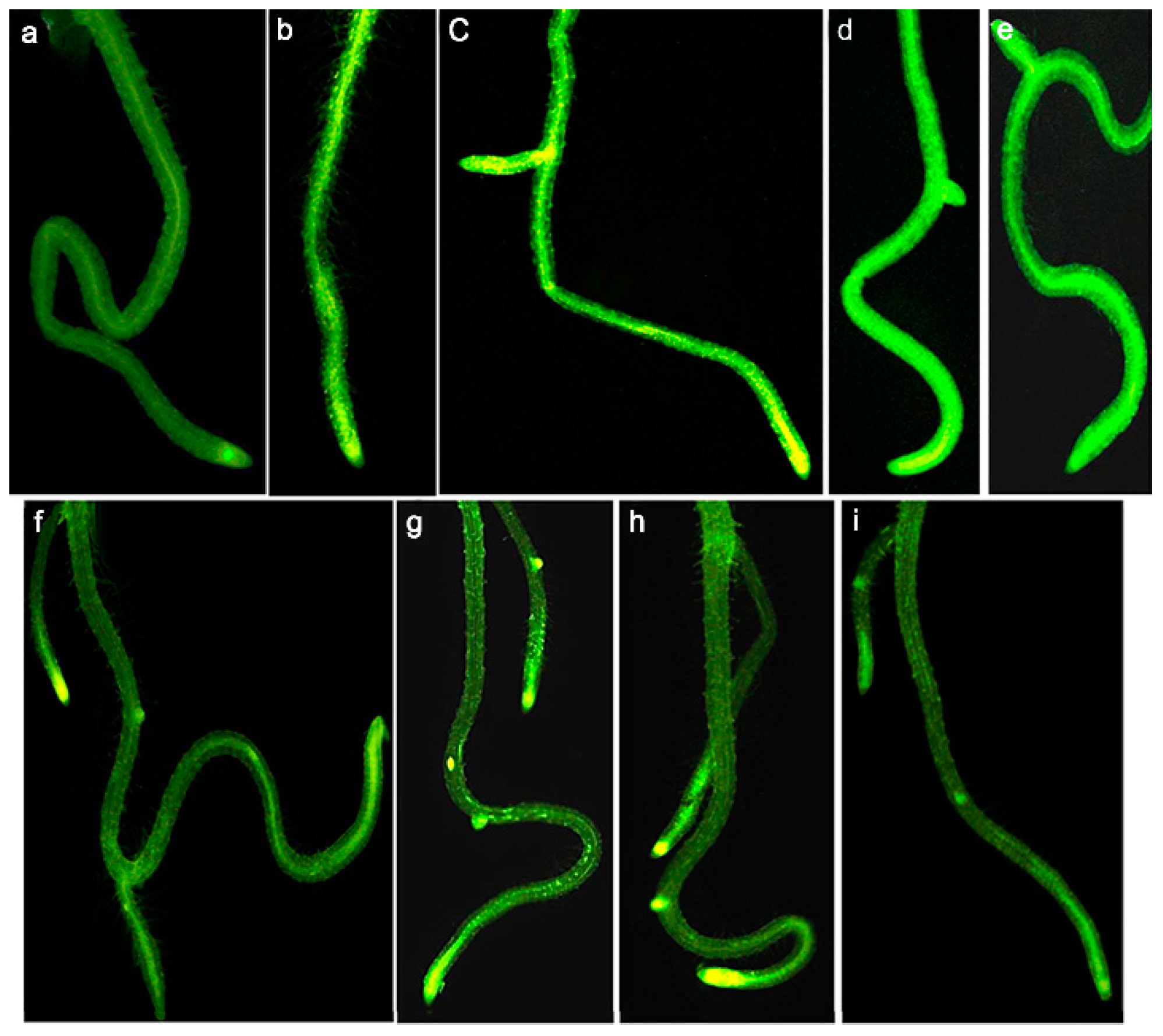
2.3. Absorption and Transport of Other Metal-Based Nanoparticles in Arabidopsis thaliana
Given their promising water solubility and small size, quantum dots (QDs) were believed to be easily absorbed by plants; this was also confirmed in the recent study of pumpkin’s physiological responses to zinc oxide quantum dots and nanoparticles [47]. The experimental results for water-dispersible CdSe/ZnSe QDs showed no significant results [48]. Using confocal fluorescence microscopy, Navarro et al. found that water-soluble CdSe/ZnS QDs with carboxyl groups were strongly adsorbed to polar/charged root surfaces but could not enter the roots. Moreover, despite a 7-day exposure period, the plant cells remained impermeable to QDs, and therefore, QDs could neither be endocytosed nor passively or actively transported through the plant root system (Figure 5), suggesting the significant effect of the surface charge of nanoparticles on their uptake by Arabidopsis. In addition to the barrier created by the plant’s cell wall, when QDs are electrostatically adsorbed on the root surface, they form bulky agglomerates, which further impedes their entry as endocytosis cannot occur [49].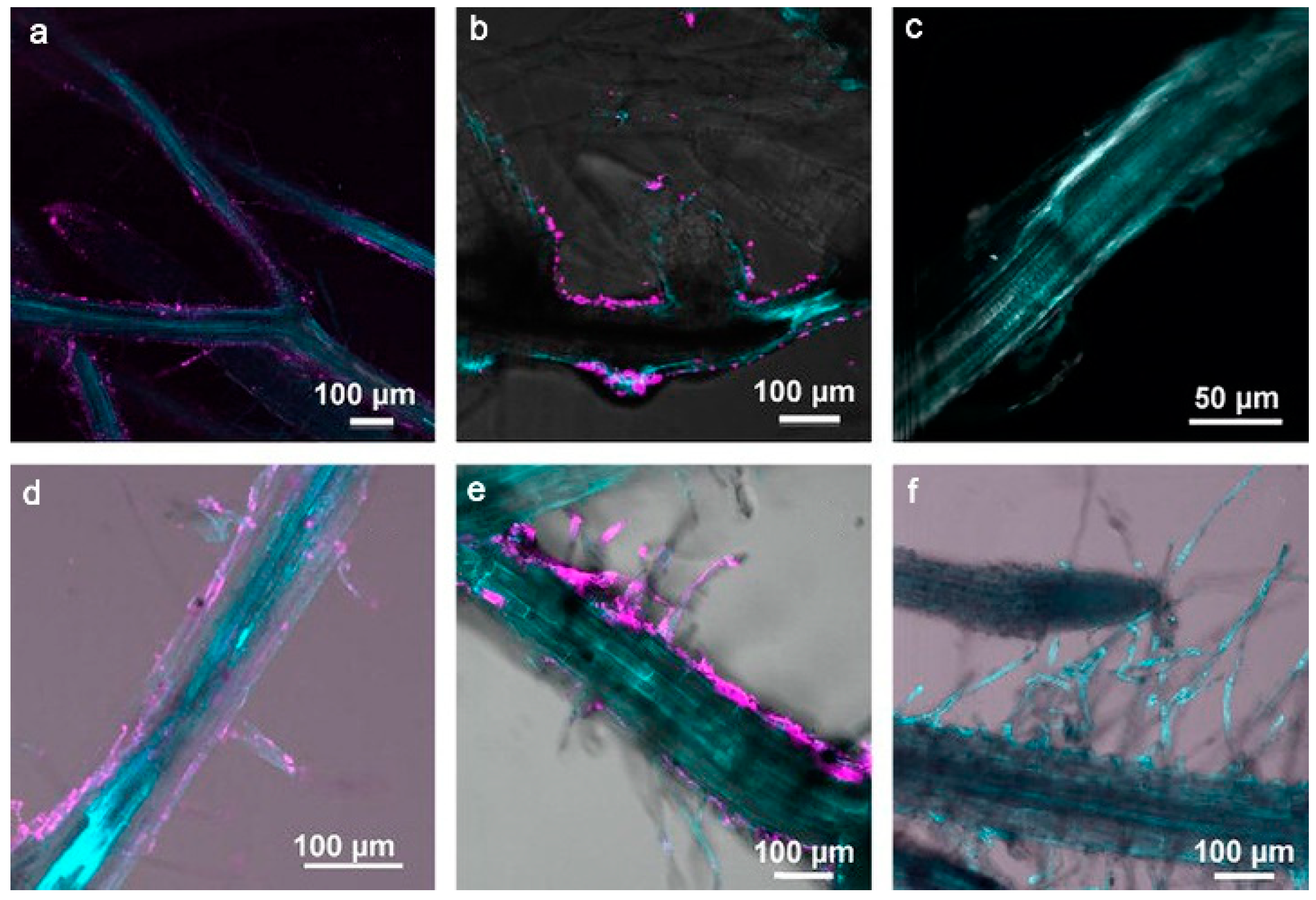
References
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36.
- Prashant, K.J.; Huang, X.; Ivan, H.E.-S.; Mostafa, A.E.-S. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2007, 41, 1578–1586.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145.
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076.
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331.
- Haghighi, M.; Teixeira da Silva, J.A. The effect of N-TiO2 on tomato, onion, and radish seed germination. J. Crop Sci. Biotechnol. 2014, 17, 221–227.
- Zhang, Z.; Ke, M.; Qu, Q.; Peijnenburg, W.; Lu, T.; Zhang, Q.; Ye, Y.; Xu, P.; Du, B.; Sun, L.; et al. Impact of copper nanoparticles and ionic copper exposure on wheat (Triticum aestivum L.) root morphology and antioxidant response. Environ. Pollut. 2018, 239, 689–697.
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.-C.; Braam, J.; Alvarez, P.J. Alvarez. developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675.
- Chen, Y.; Chen, H.; Zheng, X.; Mu, H. The impacts of silver nanoparticles and silver ions on wastewater biological phosphorous removal and the mechanisms. J. Hazard. Mater. 2012, 239–240, 88–94.
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717.
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302.
- Corredor, E.; Testillano, P.S.; Coronado, M.J.; Gonzalez-Melendi, P.; Fernandez-Pacheco, R.; Marquina, C.; Ibarra, M.R.; de la Fuente, J.M.; Rubiales, D.; Perez-de-Luque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant Biol. 2009, 9, 45.
- Shams, G.; Ranjbar, M.; Amiri, A. Effect of silver nanoparticles on concentration of silver heavy element and growth indexes in cucumber (Cucumis sativus L. negeen). J. Nanopart. Res. 2013, 15, 1630.
- Daohui Lin, B.X. Root Uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585.
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67.
- Zhao, L.; Sun, Y.; Hernandez-Viezcas, J.A.; Servin, A.D.; Hong, J.; Niu, G.; Peralta-Videa, J.R.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: A life cycle study. J. Agric. Food Chem. 2013, 61, 11945–11951.
- Matthed, D.; Whiteside, K.K.T.; Peter, R.A. The brighter side of soils: Quantum dots track organic nitrogen through fungi and plants. Ecology 2009, 90, 100–108.
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 33.
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033.
- Zhai, G.; Walters, K.S.; Peate, D.W.; Alvarez, P.J.; Schnoor, J.L. Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ. Sci. Technol. Lett. 2014, 1, 146–151.
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.; Bruce, N.C. Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE 2014, 9, e93793.
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498.
- Kohan-Baghkheirati, E.; Geisler-Lee, J. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 2015, 5, 436–467.
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of toxicity of Ag nanoparticles in comparison to bulk and ionic Ag on mussel hemocytes and gill cells. PLoS ONE 2015, 10, e0129039.
- Scherer, M.D.; Sposito, J.C.V.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.C.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Goncalves, D.A.; Wender, H.; et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total Environ. 2019, 660, 459–467.
- Koo, Y.; Lukianova-Hleb, E.Y.; Pan, J.; Thompson, S.M.; Lapotko, D.O.; Braam, J. In planta response of arabidopsis to photothermal impact mediated by gold nanoparticles. Small 2016, 12, 623–630.
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2013, 7, 323–337.
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle uptake in plants: Gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ. Sci. Technol. 2017, 51, 8682–8691.
- Milewska-Hendel, A.; Zubko, M.; Stroz, D.; Kurczynska, E.U. Effect of nanoparticles surface charge on the Arabidopsis thaliana (L.) roots development and their movement into the root cells and protoplasts. Int. J. Mol. Sci. 2019, 20, 1650.
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998.
- Mir, A.H.; Qamar, A.; Qadir, I.; Naqvi, A.H.; Begum, R. Accumulation and trafficking of zinc oxide nanoparticles in an invertebrate model, Bombyx mori, with insights on their effects on immuno-competent cells. Sci. Rep. 2020, 10, 1617.
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 17029.
- Jain, A.; Sinilal, B.; Dhandapani, G.; Meagher, R.B.; Sahi, S.V. Effects of deficiency and excess of zinc on morphophysiological traits and spatiotemporal regulation of zinc-responsive genes reveal incidence of cross talk between micro- and macronutrients. Environ. Sci. Technol. 2013, 47, 5327–5335.
- Hussain, D.; Haydon, M.J.; Wang, Y.; Wong, E.; Sherson, S.M.; Young, J.; Camakaris, J.; Harper, J.F.; Cobbett, C.S. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 2004, 16, 1327–1339.
- Nair, P.M.G.; Chung, I.M. Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 2017, 575, 187–198.
- Jia, H.; Chen, S.; Wang, X.; Shi, C.; Liu, K.; Zhang, S.; Li, J. Copper oxide nanoparticles alter cellular morphology via disturbing the actin cytoskeleton dynamics in Arabidopsis roots. Nanotoxicology 2020, 14, 127–144.
- Wang, Z.; Xu, L.; Zhao, J.; Wang, X.; White, J.C.; Xing, B. CuO Nanoparticle interaction with Arabidopsis thaliana: Toxicity, parent-progeny transfer, and gene expression. Environ. Sci. Technol. 2016, 50, 6008–6016.
- Hayat, S.; Pichtel, J.; Faizan, M. Sustainable agriculture reviews 41 nanotechnology for plant growth and development. In Nanotechnology for Plant Growth and Development; Springer: Berlin/Heidelberg, Germany, 2020.
- Garcia-Sanchez, S.; Bernales, I.; Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom. 2015, 16, 341.
- Wang, S.; Kurepa, J.; Smalle, J.A. Ultra-small TiO2 nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ. 2011, 34, 811–820.
- Ma, C.; Chhikara, S.; Xing, B.; Musante, C.; White, J.C.; Dhankher, O.P. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain. Chem. Eng. 2013, 1, 768–778.
- Birbaum, K.; Brogioli, R.; Schellenberg, M.; Martinoia, E.; Wendelin, E.; Stark, J.W.; Detlef, G.; Ludwig, K.L. No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ. Sci. Technol. 2010, 44, 8718–8723.
- Martha, L.L.-M.; Rose, G.D.L.; Jose, A.H.-V.; Castillo-Michel, H.; Botez, C.E.; Jose, R.P.-V.; Jorge, L.G.-T. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320.
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.J.; et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456.
- Zhang, P.; Ma, Y.; Zhang, Z.; He, X.; Zhang, J.; Guo, Z.; Tai, R.; Zhao, Y.; Chai, Z. Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano 2012, 6, 9943–9950.
- Xu, X.; Zhao, C.; Qian, K.; Sun, M.; Hao, Y.; Han, L.; Wang, C.; Ma, C.; White, J.C.; Xing, B. Physiological responses of pumpkin to zinc oxide quantum dots and nanoparticles. Environ. Pollut. 2022, 296, 118723.
- Kolackova, M.; Moulick, A.; Kopel, P.; Dvorak, M.; Adam, V.; Klejdus, B.; Huska, D. Antioxidant, gene expression and metabolomics fingerprint analysis of Arabidopsis thaliana treated by foliar spraying of ZnSe quantum dots and their growth inhibition of Agrobacterium tumefaciens. J. Hazard. Mater. 2019, 365, 932–941.
- Navarro, D.A.; Bisson, M.A.; Aga, D.S. Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J. Hazard. Mater. 2012, 211–212, 427–435.
- Laaveri, T.; Sterne, J.; Rombo, L.; Kantele, A. Systematic review of loperamide: No proof of antibiotics being superior to loperamide in treatment of mild/moderate travellers’ diarrhoea. Travel Med. Infect. Dis. 2016, 14, 299–312.
- Shen, Y.; Borgatta, J.; Ma, C.; Singh, G.; Tamez, C.; Schultes, N.P.; Zhang, Z.; Dhankher, O.P.; Elmer, W.H.; He, L.; et al. Role of foliar biointerface properties and nanomaterial chemistry in controlling Cu transfer into wild-type and mutant Arabidopsis thaliana leaf tissue. J. Agric. Food Chem. 2022, 70, 4267–4278.
- He, J.; Zhang, L.; He, S.Y.; Ryser, E.T.; Li, H.; Zhang, W. Stomata facilitate foliar sorption of silver nanoparticles by Arabidopsis thaliana. Environ. Pollut. 2022, 292, 118448.
