Pancreatic cancer is the fourth leading cause of cancer-related mortality worldwide, with a five-year survival rate of less than 8% [1]. It can be classed into endocrine and exocrine tumours with PDAC, a form of exocrine pancreatic cancer, the most common form. The high mortality rate associated with PDAC is primarily due to delayed diagnosis and tumour resistance to chemotherapy [2]. A broad range of non-specific symptoms are associated with PDAC such as abdominal pain, jaundice, dry/itchy skin, steatorrhoea, and bilirubinuria. Surgery is the primary treatment option, with chemotherapy being administered as adjuvant therapy in select cases. Resistance to chemotherapy has become a critical problem in the treatment of PDAC, with most patients displaying resistance patterns [10].
- pancreatic ductal adenocarcinoma
- chemotherapy
- chemoresistance
- immunotherapy
- synthetic lethality
- clinical trials
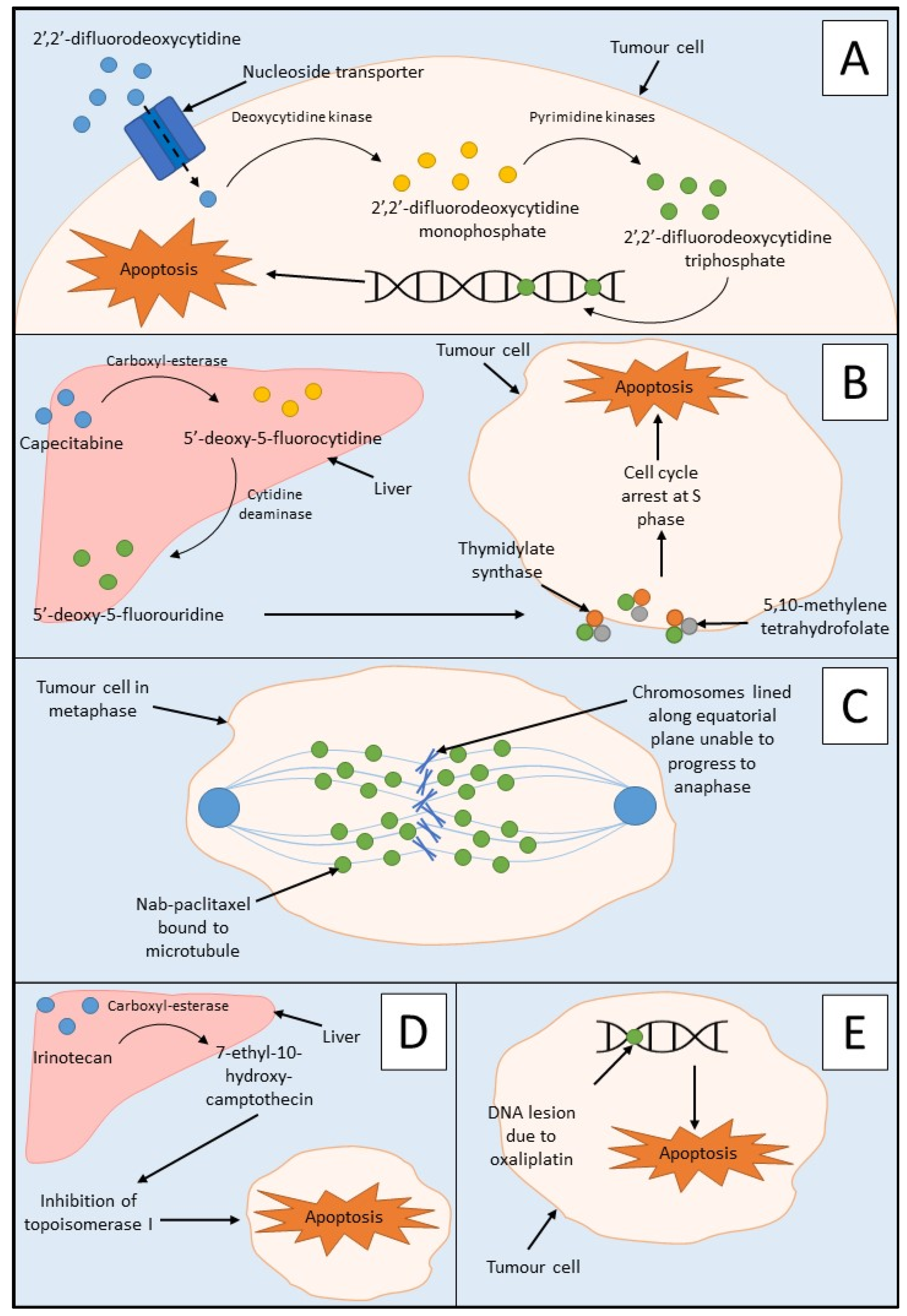
1. Current Approved Therapies
Chemotherapy
2. Targeted Therapeutic Approaches
2.1. Synthetic Lethality
Synthetic lethality is used to define an interaction between two genes in which a mutation of one is viable; however, a mutation of both leads to cell death, as outlined in Figure 2A [31,32][12][13]. It can also involve a mutation only being synthetically lethal if it is combined with another specific mutation. These synthetically lethal interactions can be described as gain-of-function alleles or loss-of-function alleles, with the most common being the latter [33][14]. Loss-of-function alleles can be categorised based on their protein product′s function; for example, they could have an essential function, be a subunit of a protein complex, or play a role in protein folding pathways. Synthetic lethality has proven successful in the treatment of BRCA1/2-mutated neoplasms which are sensitive to poly(ADP-ribose) polymerase (PARP) inhibitors [34][15]. PARP is an enzyme which is involved in the repair of single-strand DNA breaks (SSBs) [32][13]. Inhibition of PARP will lead to irreparable SSBs and, therefore, a one-ended double-stranded DNA break (DSB) via the collapse of a replication fork. Neoplasms containing a loss-of-function mutation of either the BRCA1 or BRCA2 gene lack homologous recombination; therefore, DSB results in cell death [32][13]. Due to this aspect, synthetic lethality is believed to be a possible route for potential anticancer drugs in the treatment of PDAC via the proteins produced by potential synthetically lethal genes with mutations [33][14]. However, commonly occurring loss-of-function mutations in PDAC such as those in CDKN2A, TP53, and SMAD4 are currently not targetable due to the many genetic aberrations, mainly point mutations, associated with each gene [35][16].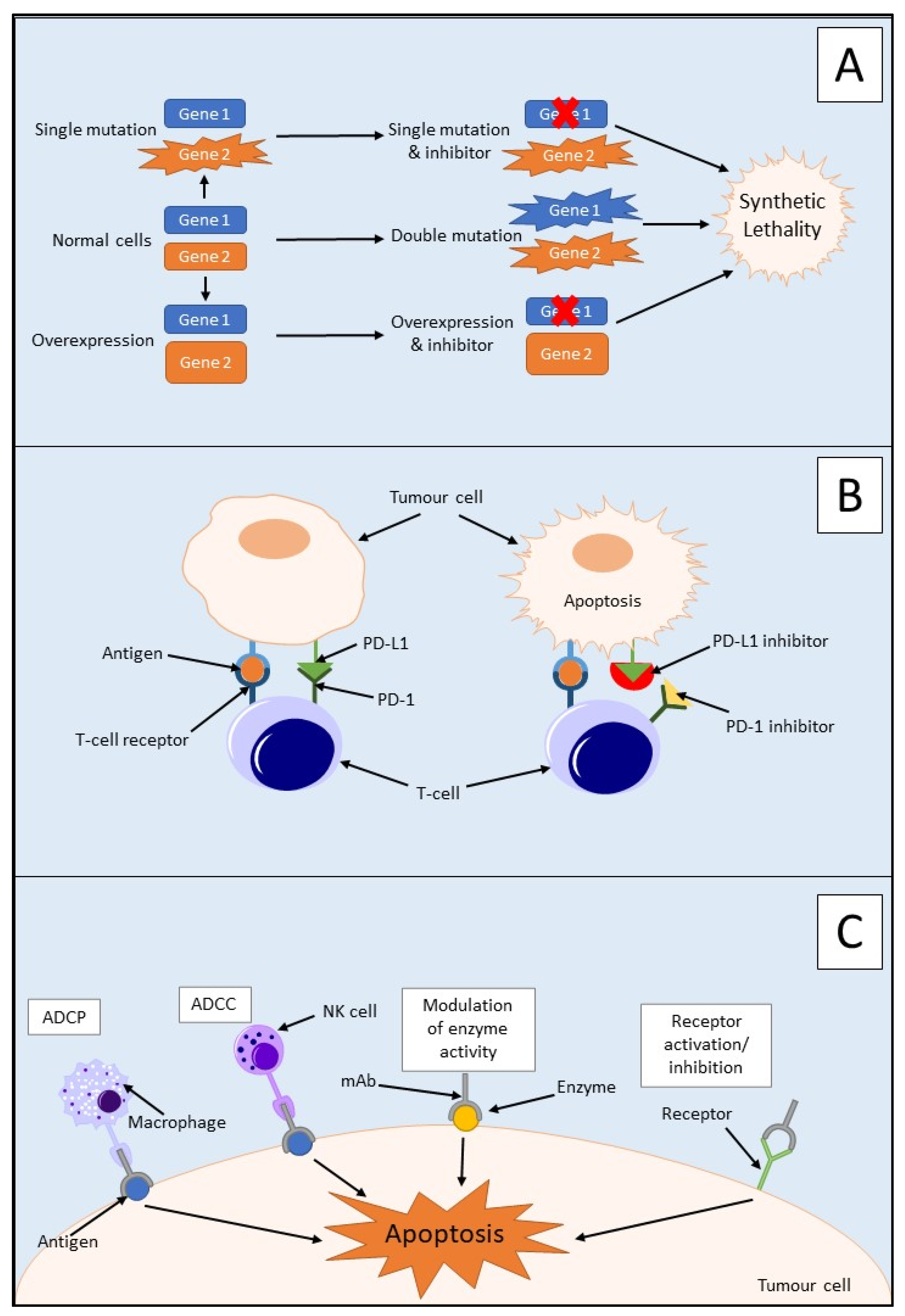
2.2. Immunotherapy
2.2.1. Immune Checkpoint Inhibitors
Immune checkpoint inhibitors are an immunotherapeutic strategy that activates the immune system to modulate the immune response to cancer [41][20]. This occurs through the stimulation of the innate and adaptive immune systems, with the main focus on the activation of T cells.
Programmed death-1 (PD-1) is an immune checkpoint protein that is activated by its ligand PD-L1 and is expressed by activated T cells [45][21]. PD-L1 is a membrane protein that is expressed on immune and tumour cells and inactivates T cells by inducing programmed cell death via heterodimer formation with CD80 [46,47][22][23]. Overexpression of PD-L1 has been detected in several cancers, including PDAC, and this overexpression is associated with advanced tumour stage and, therefore, a poorer prognosis for patients [45,48][21][24]. PD-L1 blockade alone has been shown to display minimal inhibition in PDAC and, therefore, is not regarded as a sufficient therapeutic target alone. This could be due to the non-immunogenic nature of PDAC or immunosuppression due to the high tumour burden [45][21].
Due to the poor success of immune checkpoint inhibitor monotherapy in PDAC, potential combination therapies are being sought to improve its efficacy. Cancer Forkhead box protein 3 (cFOXP3) is an upregulated protein in PDAC and plays a vital role with regulatory T cells and immune evasion in cancer [49][25]. It achieves this through the recruitment of cFOXP3 positive regulatory T cells to the site of malignancy via upregulation of C–C chemokine ligand 5 (CCL5). PD-L1 expression in PDAC has been shown to coexist with regulatory T-cell infiltration of tumours. This suggests a possible link between PD-L1 and cFOXP3 expression [49][25].
32.2.2. Monoclonal Antibodies
32.2.3. Adoptive Cell Transfer
Adoptive cell transfer is the transfer of immune cells into a patient as a form of therapy to improve the patient’s immune system. The use of natural killer cells as a form of adoptive cell transfer therapy has recently become a point of interest in the field of immunotherapy [56][27]. This is due to their ability to target and eliminate tumour cells via cytotoxic mechanisms and the role they play in inducing an adaptive immune response. In the pre-cancerous stages of PDAC, there is a loss of natural killer cells due to mutations in KRAS. This could be due to their involvement in the initiation and progression of PDAC.32.2.4. Therapeutic Vaccination
Any tumour cells remaining post-surgery/treatment could lead to a relapse of the PDAC tumour [57][28]. Therefore, vaccination yields a possible approach to target remnant tumour cells via activation of the immune system toward tumour-associated antigens. The whole tumour cell lysate was utilised by processing murine PDAC tumour membranes to enable them to be opsonised by naturally occurring human IgG antibodies [57][28]. This can stimulate the immune system to target tumour associated antigens, in this case, galactose-alpha-1,3-galactose (α-gal). An immune response was mounted against the PDAC tumour lysate vaccine, resulting in antitumour properties in murine models. A statistically significant median OS was observed with mice treated with PDAC tumour lysate and α-gal of 95.0 days (95% CI, 69–95), compared with the untreated control mice of 40.0 days (95% CI, 35–45) (p < 0.01). This shows a potential route of treatment for PDAC which could be utilised in the future [57][28].3.3. Ongoing Clinical Trials
2.3. Ongoing Clinical Trials
At present, there are a plethora of clinical trials recruiting patients that involve the treatment of PDAC. Many of these studies involve the utilisation of immunotherapies, in particular, monoclonal antibodies in combination with another form of therapy such as conventional chemotherapy. An example of this is a sstudy currently recruiting in the Cancer Centre at Johns Hopkins University which aims to determine the effects of pembrolizumab which could be given in combination with defactinib in patients with resectable PDAC as a form of neoadjuvant or adjuvant therapy [50][29]. Pembrolizumab is a monoclonal antibody directed towards PD-1, and this may or may not be administered intravenously alongside defactinib, a focal adhesion kinase inhibitor. Focal adhesion kinase is a non-receptor tyrosine kinase that is involved in cell scaffolding and signalling at the sites of integrin clustering on the cell membrane [59][30]. The effects of these drugs will be determined in combination with standard neoadjuvant and adjuvant chemotherapy regimens such as gemcitabine [50][29]. This sItudy will determine if the tumour microenvironment can be reprogrammed via targeting focal adhesion kinase post-chemotherapy and, therefore, potentiate anti-PD-1 effects to halt cancer progression [50][29].
References
- Salinas-Miranda, E.; Deniffel, D.; Dong, X.; Healy, G.M.; Khalvati, F.; O’Kane, G.M.; Knox, J.; Bathe, O.F.; Baracos, V.E.; Gallinger, S.; et al. Prognostic value of early changes in CT-measured body composition in patients receiving chemotherapy for unresectable pancreatic cancer. Eur. Radiol. 2021, 31, 8662–8670.
- Hamed, S.S.; Straubinger, R.M.; Jusko, W.J. Pharmacodynamic modeling of cell cycle and apoptotic effects of gemcitabine on pancreatic adenocarcinoma cells. Cancer Chemother. Pharmacol. 2013, 72, 553–563.
- NICE. Pancreatic Cancer in Adults: Diagnosis and Management; NICE: London, UK, 2018.
- Gelibter, A.; Malaguti, P.; Di Cosimo, S.; Bria, E.; Ruggeri, E.M.; Carlini, P.; Carboni, F.; Ettorre, G.M.; Pellicciotta, M.; Giannarelli, D.; et al. Fixed dose-rate gemcitabine infusion as first-line treatment for advanced-stage carcinoma of the pancreas and biliary tree. Cancer 2005, 104, 1237–1245.
- Schellens, J.H. Capecitabine. Oncologist 2007, 12, 152–155.
- Köhne, C.H.; Peters, G.J. UFT: Mechanism of drug action. Oncology 2000, 14, 13–18.
- Shen, Z.T.; Zhou, H.; Li, A.M.; Ji, X.Q.; Jiang, C.C.; Yuan, X.; Li, B.; Zhu, X.X.; Huang, G.C. Clinical outcomes and prognostic factors of stereotactic body radiation therapy combined with gemcitabine plus capecitabine for locally advanced unresectable pancreatic cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 417–428.
- Chen, N.; Brachmann, C.; Liu, X.; Pierce, D.W.; Dey, J.; Kerwin, W.S.; Li, Y.; Zhou, S.; Hou, S.; Carleton, M.; et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother. Pharmacol. 2015, 76, 699–712.
- De Vita, F.; Ventriglia, J.; Febbraro, A.; Laterza, M.M.; Fabozzi, A.; Savastano, B.; Petrillo, A.; Diana, A.; Giordano, G.; Troiani, T.; et al. NAB-paclitaxel and gemcitabine in metastatic pancreatic ductal adenocarcinoma (PDAC): From clinical trials to clinical practice. BMC Cancer 2016, 16, 709.
- Deyme, L.; Barbolosi, D.; Gattacceca, F. Population pharmacokinetics of FOLFIRINOX: A review of studies and parameters. Cancer Chemother. Pharmacol. 2019, 83, 27–42.
- Khushman, M.; Dempsey, N.; Maldonado, J.C.; Loaiza-Bonilla, A.; Velez, M.; Carcas, L.; Dammrich, D.; Hurtado-Cordovi, J.; Parajuli, R.; Pollack, T.; et al. Full dose neoadjuvant FOLFIRINOX is associated with prolonged survival in patients with locally advanced pancreatic adenocarcinoma. Pancreatology 2015, 15, 667–673.
- O’Neil, N.J.; Bailey, M.L.; Hieter, P. Synthetic lethality and cancer. Nat. Rev. Genet. 2017, 18, 613–623.
- Helleday, T. The underlying mechanism for the PARP and BRCA synthetic lethality: Clearing up the misunderstandings. Mol. Oncol. 2011, 5, 387–393.
- Kaelin, W.G. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 2005, 5, 689–698.
- Hu, Y.; Guo, M. Synthetic lethality strategies: Beyond BRCA1/2 mutations in pancreatic cancer. Cancer Sci. 2020, 111, 3111–3121.
- Jones, S.N.; Zhang, X.; Parsons, D.W.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science 2008, 321, 1801–1806.
- Fan, J.Q.; Wang, M.F.; Chen, H.L.; Shang, D.; Das, J.K.; Song, J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol. Cancer 2020, 19, 32.
- Dean-Colomb, W.; Esteva, F.J. Her2-positive breast cancer: Herceptin and beyond. Eur. J. Cancer 2008, 44, 2806–2812.
- Weiner, G.J. Rituximab: Mechanism of action. Semin. Hematol. 2010, 47, 115–123.
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249.
- Feng, M.; Xiong, G.; Cao, Z.; Yang, G.; Zheng, S.; Song, X.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017, 407, 57–65.
- Wang, X.; Li, X.; Wei, X.; Jiang, H.; Lan, C.; Yang, S.; Wang, H.; Yang, Y.; Tian, C.; Xu, Z.; et al. PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Signal Transduct. Target. Ther. 2020, 5, 38.
- Zhao, Y.; Lee, C.K.; Lin, C.H.; Gassen, R.B.; Xu, X.; Huang, Z.; Xiao, C.; Bonorino, C.; Lu, L.F.; Bui, J.D.; et al. PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways. Immunity 2019, 51, 1059–1073.e1059.
- Geng, L.; Huang, D.; Liu, J.; Qian, Y.; Deng, J.; Li, D.; Hu, Z.; Zhang, J.; Jiang, G.; Zheng, S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J. Cancer Res. Clin. Oncol. 2008, 134, 1021–1027.
- Wang, X.; Lang, M.; Zhao, T.; Feng, X.; Zheng, C.; Huang, C.; Hao, J.; Dong, J.; Luo, L.; Li, X.; et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3. Oncogene 2017, 36, 3048–3058.
- Maron, R.; Schechter, B.; Nataraj, N.B.; Ghosh, S.; Romaniello, D.; Marrocco, I.; Noronha, A.; Carvalho, S.; Yarden, Y.; Sela, M. Inhibition of a pancreatic cancer model by cooperative pairs of clinically approved and experimental antibodies. Biochem. Biophys. Res. Commun. 2019, 513, 219–225.
- Hu, S.; Yang, J.; Shangguan, J.; Eresen, A.; Li, Y.; Ma, Q.; Yaghmai, V.; Velichko, Y.; Hu, C.; Zhang, Z. Natural killer cell-based adoptive transfer immunotherapy for pancreatic ductal adenocarcinoma in a. Am. J. Cancer Res. 2019, 9, 1757–1765.
- Furukawa, K.; Tanemura, M.; Miyoshi, E.; Eguchi, H.; Nagano, H.; Matsunami, K.; Nagaoka, S.; Yamada, D.; Asaoka, T.; Noda, T.; et al. A practical approach to pancreatic cancer immunotherapy using resected tumor lysate vaccines processed to express α-gal epitopes. PLoS ONE 2017, 12, e0184901.
- Zheng, L. Study of Pembrolizumab with or without Defa Actinib Following Chemotherapy as a Neoadjuvant and Adjuvant Treatment for Resectable Pancreatic Ductal Adenocarcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03727880?recrs=ab&cond=pdac&draw=2&rank=1 (accessed on 11 August 2021).
- McLean, G.W.; Carragher, N.O.; Avizienyte, E.; Evans, J.; Brunton, V.G.; Frame, M.C. The role of focal-adhesion kinase in cancer—A new therapeutic opportunity. Nat. Rev. Cancer 2005, 5, 505–515.
