Urolithin尿石素 A (Uro A) is a dietary metabolite of the intestinal microbiota following the ingestion of plant-based food ingredients ellagitannins and ellagic acid in mammals. Accumulating studies have reported its multiple potential health benefits in a broad range of diseases, including cardiovascular disease, cancer, cognitive impairment, and diabetes.是哺乳动物摄入植物性食物成分鞣花单宁和鞣花酸后肠道微生物群的饮食代谢物。越来越多的研究报告了它对多种疾病的多种潜在健康益处,包括心血管疾病、癌症、认知障碍和糖尿病。特别是,Uro A 通过直接口服给药是安全的,并且没有遗传毒性。胰腺通过分泌消化酶和激素在调节能量消耗和新陈代谢方面发挥核心作用。许多病理生理因素,如炎症、线粒体自噬缺陷和内质网应激,会对胰腺产生负面影响,导致胰腺疾病,包括胰腺炎、胰腺癌和糖尿病。
1. Reduces the Expression of Pancreatic Inflammatory Factors减少胰腺炎症因子的表达
The胰腺炎性微环境导致胰腺炎,是内分泌功能下降的主要原因[ inflammatory83 microenvironment]。一些研究人员认为,如果 of the pancreas led to pancreatitis and was the main reason for the decline in endocrine function [1]. Some researchers suggested that if β
cells细胞表达高水平的 would express high levels of NF-κB
signaling marking, cells’ proliferative and regenerative potential were reduced. The NF-κB-expressed β cells also emerged with a premature upregulation of 信号标记,细胞的增殖和再生潜力就会降低。表达 NF-κB 的 β 细胞也出现了socs2,的过早上调,这是一种抑制增殖的基因 a[ gene84 that]。已广泛报道 inhibits proliferation [2]. It has been widely reported that EA
can inhibit pancreatic inflammation (Table可抑制胰腺炎症(表 1). In an)。在自发性慢性胰腺炎的实验模型中,雄性 experimental model of spontaneous chronic pancreatitis, male Wistar Bonn/Kobori
rats were fed a diet supplemented with EA (大鼠被喂食添加 EA(100 mg/kg
body weight/day) for ten weeks. They found that EA attenuated pancreatic inflammation and fibrosis by increasing pancreatic weight and decreasing MPO activity (a neutrophil infiltration index), collagen content, transforming growth factor体重/天)的饮食十周。他们发现 EA 通过增加胰腺重量和降低 MPO 活性(中性粒细胞浸润指数)、胶原蛋白含量、转化生长因子-β1 (TGF-β1)
expression, activated pancreatic stellate cells (PSCs), and ED-1-positive cells表达、活化胰腺星状细胞 (PSC) 和 ED- 来减轻胰腺炎症和纤维化。 [3].1 Masamune阳性细胞 et[ al.85 also]。正宗等人。还报道 reported that EA inhibited the production of monocyte chemoattractant protein-EA 抑制 PSC 中单核细胞趋化蛋白 1 (MCP-1)
and activation of activator protein-的产生和激活蛋白 1 (AP-1)
and和 MAPK
in PSCs, all induced by interleukin 的激活,所有这些均由白细胞介素 (IL)-1β
and和 TNF-α
[4].诱导 Meanwhile,[ 86]。同时,EA
inhibited抑制 PDGF-BB
-induced tyrosine phosphorylation of 诱导的 PDGF P
-receptors and the downstream ERK and Akt activation in PSCs. In particular, EA inhibited ROS production in PSCs in response to 受体酪氨酸磷酸化以及 PSC 中下游 ERK 和 Akt 的活化。特别是,EA 抑制 PSC 中响应 TGF-β1
or platelet-derived growth factor 或血小板衍生生长因子 (PDGF)
的 ROS [5].产生 [ 87 ]。
Table
Potential effects and mechanisms of urolithin A (Uro A) and its precursor compounds EA on pancreatic diseases.
| Disease Model疾病模型 |
Treatment治疗 |
Metabolic Response代谢反应 |
Ref.参考。 |
| Spontaneous CP雄性 in male Wistar Bonn/Kobori rats大鼠的自发性 CP |
100 mg/kg BW/day天与 orally administered with EA for 10 weeksEA 一起口服给药 10 周 |
-
Attenuated pancreatic inflammation and fibrosis减轻胰腺炎症和纤维化
-
Increased pancreatic weight胰腺重量增加
-
Decreased MPO activity, collagen content, 活性、胶原蛋白含量、TGF-β1 expression; activated PSCs and ED-1-positive cells表达降低;活化的 PSC 和 ED-1 阳性细胞
|
[3][ 85 ] |
| 从大鼠胰腺组织中分离出 PSCs were isolated from rat pancreas tissue to culture activated, myofibroblast-like phenotype,以培养活化的肌成纤维细胞样表型 |
1–25 μg/mL EA |
-
Inhibited抑制 PSCs’ proliferation and migration 的增殖和迁移
-
Inhibited抑制 MCP-1 production and the expression of smooth muscle actin and collagen genes的产生和平滑肌肌动蛋白和胶原基因的表达
-
Inhibited the tyrosine抑制 phosphorylation of PDGF P-receptor 受体的酪氨酸磷酸化
-
Inhibited抑制 the activation of Akt and MAPKsAkt 和 MAPK 的激活
|
[4][ 86 ] |
| L-arginine精氨酸诱导大鼠 induced AP in rats |
85 mg/kg orally与 administered with EA 一起口服 |
-
Decreased降低 TOS levels水平
-
Increased增加 TAC levels水平
-
Decreased降低 TNF-α, IL-1β, and、IL-1β 和 IL-6 serum levels血清水平
-
Healed inflammatory and oxidative stress治愈炎症和氧化应激
|
[5][ 87 ] |
| MIN6使用 β-cell inflammations were induced using 25 mM glucose and 葡萄糖和 0.5 mM palmitic acid棕榈酸诱导 MIN6 β 细胞炎症 |
Uro 尿素A |
|
[6][ 88 ] |
| Alcohol-associated CP in C56BL6/J mice小鼠中的酒精相关 CP |
Administered在酒精相关 during the last 3 weeks of alcohol-associated CP inductionCP 诱导的最后 3 周内给药 |
|
[7][ 89 ] |
| 通过 HFDM in male 和腹膜内 STZ 注射实现雄性 C57BL/6 mice was achieved by a HFD and intraperitoneal STZ injections小鼠的 DM |
50 mg/kg BW/day orally天与 administered with Uro A for 8 weeks一起口服给药 8 周 |
|
[8][ 90 ] |
| Human人 PDAC cell lines; PDAC mice were achieved by injecting 细胞系;PDAC 小鼠是通过将 PANC1 cells into the flank of 6-week-old 细胞注射到 6 周大的 Fox1-nu/nu mice小鼠的侧腹来实现的 |
0–100 μM微米; 20 mg/kg BW/day (5 days/week) orally administered with 天(5 天/周)与 Uro A 一起口服 |
-
Inhibited the proliferation and migration of 抑制PDAC cells细胞的增殖和迁移
-
Enhanced通过下调 apoptosis by down-regulating the PI3K/AKT/mTOR pathway通路增强细胞凋亡
-
Inhibited抑制 the PDK1/AKT/mTOR pathway通路
-
Reduced减少免疫抑制性 immunosuppressive TAMs and regulatory T cellsTAM 和调节性 T 细胞
|
[9][ 91 ] |
| PKT ((Ptf1a cre/+; ;LSL-Kras G12D; ;Tgfbr2 fl/fl))小鼠,一种具有攻击性的基因工程 mice, an aggressive genetically engineered PDAC mouse modelPDAC 小鼠模型 |
Orally口服 administered with Uro A for 5 weeks5 周 |
-
Inhibited抑制 AKT, PS6K, and、PS6K 和 STAT3 signaling信号传导
-
Reduced减少 the Ki67-positive tumor cellsKi67 阳性肿瘤细胞
-
Increased cleaved裂解的 caspase-3 expression表达增加
|
[10][ 92 ] |
| Neonatal新生 STZ-induced non-obese 诱导的非肥胖 T2DM rats大鼠 |
25–100 mg/kg BW orally与 administered with EA 一起口服给药 |
|
[11][ 93 ] |
Although尽管 EA
had many promising developments, it was poorly absorbed in the human gut, limiting its anti-inflammatory effects. As mentioned above, 有许多有希望的发展,但它在人体肠道中的吸收很差,限制了它的抗炎作用。如上所述,EA
was metabolized by microorganisms into a series of downstream compounds, such as 被微生物代谢成一系列下游化合物,如 Uro A
[12].[ A5 well-known]。暴露于 effect of preclinical models exposed to Uro A
was the的临床前模型的一个众所周知的效果是有害炎症反应的减弱 [ attenuation94 of harmful inflammatory responses [13]. ]。Uro A
showed显示出比 more potent anti-inflammatory properties than EA or ETs, suggesting that it might be the main compound for treating AP or CP (TableEA 或 ET 更有效的抗炎特性,表明它可能是治疗 AP 或 CP 的主要化合物(表 1).)。首次报道抗炎作用可降低急性结肠炎大鼠炎症标志物 The anti-inflammatory effects were first reported to reduce the mRNA and proteinCOX-2 的 mRNA 和蛋白质水平 [ levels of inflammatory marker COX-2 in rats with acute colitis [14]. Zhang et95]。张等人。首次报道 al. had firstly reported that Uro A
inhibited the thioredoxin-interacting protein (TXNIP)/Nod-like receptor family pyrin domain containing 通过调节 AMPK 抑制 MIN6 β 细胞中含有 3 (NLRP3)/IL-1β
inflammation signal in MIN6 β cells by modulating AMPK (Figure炎症信号的硫氧还蛋白相互作用蛋白 (TXNIP)/Nod 样受体家族 pyrin 结构域(图 1)2)[ [6].88 Finally, they testified]。最后,他们证明 that Uro A
also down-regulated the protein kinase 还下调蛋白激酶 RNA (PKR)
-like ER kinase 样 ER 激酶 (PERK)
and promoted AMPK phosphorylation并促进 AMPK 磷酸化 [15].[ The96 latest research showed that ]。最新研究表明,Uro A
can attenuate the severity of alcohol-associated chronic pancreatitis (ACP) in可以通过调节 PI3K/AKT/mTOR 信号轴来减轻 C56BL6/J
mice by regulating the小鼠酒精相关慢性胰腺炎 (ACP) 的严重程度 [ PI3K/AKT/mTOR89 signaling axis [7].
]。
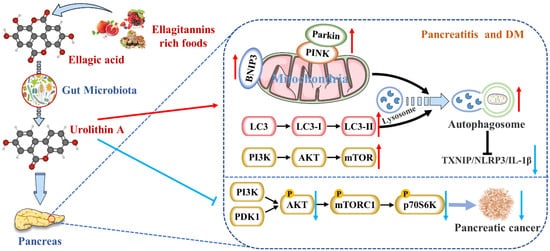
Figure图 12. Uro A is在哺乳动物摄入 metabolized by gut microbiota after ingestion of ETs and EA in mammals and has multiple potential health benefits. ET 和 EA 后被肠道微生物群代谢,具有多种潜在的健康益处。Uro A can attenuate pancreatic diseases by inhibiting inflammatory signaling pathways, activating autophagy, maintaining the mitochondrial function, and improving the immune microenvironment.可通过抑制炎症信号通路、激活自噬、维持线粒体功能和改善免疫微环境来减轻胰腺疾病。
Nevertheless, studies on reducing pancreatic inflammation by Uro A had only been verified in animals and cells without clinical studies. The upstream mediators of Uro A’s anti-inflammatory effects, including the NF-κB and AhR-Nrf2 pathways, were mainly studied in vitro [16]. Nevertheless, the mechanisms of Uro A action in the context of inflammation seemed to vary with tissues and conditions. Hence, the differences in Uro A’s mitigation degree and mechanism on AP and CP need to be further explored.
2. Activates Autophagy and Maintains Mitochondrial Function in the Pancreas
Mitochond然而,Ur
ial damage, such as the lo
ss of mitochondrial DNA (mt DNA) integrity, the alteration of mitochondrial morphology, A 减少胰腺炎症的研究仅在动物和细胞中得到验证,而没有进行临床研究。Uro A 抗炎作用的上游介质,包括 NF-κB 和 AhR-Nrf2 通路,主要在体外进行了研究 [ and36 dysfuncti]。然而,Uro
n, can lead to cellular senescence and apoptosis A 在炎症背景下的作用机制似乎因组织和条件而异。因此,Uro A 对 AP 和 [17].CP On的缓解程度和机制的差异需要进一步探讨。
2. 激活自噬并维持胰腺中的线粒体功能
线粒体损伤,例如线粒体 theDNA one hand, mitochondria acted as both(mt DNA) 完整性的丧失、线粒体形态的改变和功能障碍,可导致细胞衰老和凋亡 [ nutrient97 sensors and signal]。一方面,线粒体在 generators for insulin secretion in β cells. Moreover, nutrients can inhibit the ATP-sensitiveβ 细胞中既充当营养传感器,又充当胰岛素分泌的信号发生器。此外,营养物质可以抑制 ATP 敏感的 K
+ (K
ATP )
channel and then enhance insulin secretion either by acting as substrates for mitochondrial ATP synthesis (the triggering pathway)通道,然后通过作为线粒体 ATP 合成的底物(触发途径)或通过调节 Ca or by regulating Ca2+ channels来增强胰岛素分泌。通道(放大途径)。另一方面,线粒体是电子传递链水平上活性氧 (
the amplifying pathway). On the other hand, mitochondria were the primary source of reactive oxygen species (ROS)
at the level of the electron transport chain so that mitochondria might be the main targets of ROS damage的主要来源,因此线粒体可能是 ROS 损伤的主要目标 [18].[ Additionally,98 many]。此外,许多研究揭示了胰腺疾病与线粒体动力学失调(包括融合和裂变)之间的因果关系 studies[ have97 revealed, a99 causal relationship between pancreatic diseases and dysregulation of mitochondrial dynamics (including fusion and fission) [17][19][20]. Thus,
mitochondrial100 damage gave rise to decreased pancreatic function. The most consistent effect of ]。因此,线粒体损伤导致胰腺功能下降。Uro A
across species,在不同物种(包括细胞、蠕虫、小鼠和人类)中最一致的效果是改善线粒体健康 [ including94]。这种益处是由功能失调的线粒体(称为选择性自噬)的清除和回收驱动的 cells,[ worms,101 mice, and humans, was improved mitochondrial health [13]. This benefit was driven by the clearance and recycling of dysfunctional mitochondria, known as selective autophagy [21]. For example, ]。例如,Uro A
increased增加了编码 the expression of mitochondrial autophagy genes LC-3B 的线粒体自噬基因lgg-1, 、pink-1, and 和pdr-1,的表达,并增加了秀丽隐杆线虫中自噬体囊泡的形成 encoding[ for29 LC-3B,]。
有趣的是,小胶质细胞中的 and formation of autophagosome vesicles in C. elegans [22].
Interestingly, Pink1
knockdown敲低消除了 in microglia eliminated Uro A-mediated reductions in Uro A 介导的 TNF-α
and increased IL-10, suggesting that 减少并增加了 IL-10,这表明 Uro A
reduces neuroinflammation通过诱导线粒体自噬来减少神经炎症 [ by34 inducing]。张等人。还证明 mitochondrial autophagyUro [23].A Zhang通过调节自噬抑制 et al. also proved that Uro A inhibited glucolipotoxicity-induced ER stress and the MIN6 β 细胞中糖脂毒性诱导的 ER 应激和 TXNIP/NLRP3/IL-1β
inflammation signal炎症信号 [ in88 MIN6 β cells by modulating autophagy [6]. Remarkably, the inhibitory effects of ]。值得注意的是,Uro A
on对 p62
were stronger than TXNIP-inhibitor verapamil的抑制作用强于 TXNIP 抑制剂维拉帕米 (
p < 0.05)
[24].[ 102 ]。在糖尿病小鼠的胰腺细胞中也报道了 Uro A
promoting促进 PINK1/Parkin
-mediated mitophagy was 介导的线粒体自噬 [ also reported in pancreatic cells of diabetic mice [8]. Therefore, 90]。因此,Uro A
restoring the correct level of mitochondrial autophagy to maintain normal mitochondrial function is highly likely to be the mechanism of 恢复正确的线粒体自噬水平以维持正常的线粒体功能极有可能是Uro A
reducing pancreatic diseases (Figure 1).
减少胰腺疾病的机制(图2)。
3. Inhibits Endoplasmic Reticulum Stress in the Pancreas抑制胰腺内质网应激
The内质网 misfolding and inhibition of protein folding in the endoplasmic reticulum (ER) lead to the aggregation of unfolded proteins, resulting in ER stress(ER) 中蛋白质折叠的错误折叠和抑制导致未折叠蛋白质的聚集,从而导致 ER 应激 [25].[ Li103 et al. showed that the ]。李等人。表明伴随蛋白质聚集体积累的ER
stress and unfolded protein response (UPR) accompanied by the accumulation of protein aggregates emerged as a significant pathway affected by aging, specifically in β cells. Simultaneously, the transcriptomic dysregulation of UPR components was linked to activating transcription factor 应激和未折叠蛋白反应(UPR)成为受衰老影响的重要途径,特别是在β细胞中。同时,UPR 成分的转录组失调与激活转录因子 6 (ATF6)
and inositol-requiring enzyme 和肌醇需要酶 1 (IRE1)
signaling pathways信号通路有关 [ [26].8 ]。ER
stress-related apoptosis lead to a reduction in β-cell proliferation and regeneration, ultimately resulting in reduced insulin secretion and increased T2DM morbidity应激相关的细胞凋亡导致 β 细胞增殖和再生减少,最终导致胰岛素分泌减少和 T2DM 发病率增加 [27].[ Therefore, maintaining transcriptional stability and reducing protein homeostasis loss during aging was crucial to recovering pancreatic function. It has been reported that 104]。因此,在衰老过程中保持转录稳定性和减少蛋白质稳态损失对于恢复胰腺功能至关重要。据报道,Uro A
suppresses可抑制胰腺 β glucolipotoxicity-induced ER stress in细胞中糖脂毒性诱导的 ER 应激 [ pancreatic88 beta]。然而,需要对 cells [6]. However, more studies are needed on Uro A
’s upstream and downstream pathways in the pancreas to improve ER stress.在胰腺中的上游和下游通路进行更多的研究,以改善 ER 应激。
4. Inhibits the Occurrence and Development of Pancreatic Tumors抑制胰腺肿瘤的发生和发展
High大量摄入富含 intakes of berries rich in ETs, including strawberries, pomegranates, and blueberries, were inversely associated with PDAC incidenceET 的浆果,包括草莓、石榴和蓝莓,与 PDAC 发病率呈负相关 [ [28].105 ]。EA
, an intestinal metabolite of ellagic tannins, inhibited multiple carcinogenic pathways activated in PDAC, such as 是鞣花单宁的一种肠道代谢产物,可抑制 PDAC 中激活的多种致癌途径,例如 COX-2
, NF-κB, and Wnt signaling, so that EA successfully arrested cell cycles and reversed epithelial to mesenchymal transition in 、NF-κB 和 Wnt 信号传导,因此 EA 成功地阻止了细胞周期并逆转了 PDAC
[29].中的上皮向间质转化 As[ a106 downstream]。作为 compound of EA, EA 的下游化合物,Uro A
showed more显示出更有效的抗氧化和抗炎特性,提高生物利用度和抗肿瘤作用 [ potent107 antioxidant]。已经证明 and anti-inflammatory properties, improving bioavailability and anti-tumor effect [30]. It has been demonstrated that the AKT 的 S473
phosphorylation磷酸化位点被 site of AKT is activated by PI3K
[31].激活 [ 108]。Uro A
treatment治疗导致 resulted in a dose-dependent reduction in phospho-PDAC 细胞系中磷酸化 AKT (p-AKT)
expression in PDAC cell lines, leading to a significant down-regulation of phospho-p表达呈剂量依赖性降低,从而导致 mTORC1 复合物调节的磷酸化 p70 S6
kinase激酶 (p-PS6K)
expression regulated by the mTORC1 complex. Therefore, 表达显着下调。因此,Uro A
inhibited the proliferation and migration of PDAC cells and enhanced apoptosis by down-regulating the P通过下调 PI3K/AKT/mTOR
pathway通路抑制 PDAC [32]细胞的增殖和迁移,增强细胞凋亡 (Figure 1).[ Furthermore,109 ](图 2)。此外,Uro A
treatment治疗还下调 also down-regulated PDK1 (the upstream target of AKT) andPDK1(AKT 的上游靶点)和 p-GSK3β
and和 p-4E-BP1
(the downstream targets of AKT), suggesting that (AKT 的下游靶点),表明 Uro A
effectively inhibited the 有效抑制 PDK1/AKT/mTOR
[33].[ 110]。Uro A
treatment治疗还减少了 also reduced immunosuppressive tumor-associated macrophages (TAMs) and regulatory T cells in the engineered PKT mouse model of PDAC. It meant that PDAC 工程化 PKT 小鼠模型中的免疫抑制性肿瘤相关巨噬细胞 (TAM) 和调节性 T 细胞。这意味着 Uro A
treatment attenuated tumor growth and prolonged survival in mice by inducing changes in the immunosuppressive microenvironment of治疗通过诱导 PDAC
[9].免疫抑制微环境的变化来减弱肿瘤生长并延长小鼠的存活时间 Srinivasan[ et91 al.]。斯里尼瓦桑等人。还指出 also pointed out that Uro A
inhibited AKT, PS6K, and抑制 AKT、PS6K 和 STAT3
signaling, thereby reducing the Ki67-positive tumor cells and increasing c信号传导,从而减少 Ki67 阳性肿瘤细胞并增加 PDAC 小鼠胰腺组织中 cleaved caspase-3
expression in的表达 [ the92 pancreatic tissues of PDAC mice [10]. These results suggest that ]。这些结果表明,Uro A
is是一种新型的 a novel inhibitor/regulator for multi-signal pathways in PDAC and has potential in the prevention and treatment of pancreatic cancer (TablePDAC 多信号通路抑制剂/调节剂,具有预防和治疗胰腺癌的潜力(表 1).)。
5. Protects Pancreatic 保护胰腺β Cells细胞
There关于 have been many studies on the ameliorative effect of Uro A
on DM and its complications. Specifically, Savi et al. first showed that 对 DM 及其并发症的改善作用已有许多研究。具体来说,Savi 等人。首次表明 Uro A
recovered cardiomyocyte contractility and calcium dynamics in diabetic cardiomyopathy 在糖尿病心肌病 (DCM)
rats大鼠中恢复了心肌细胞的收缩力和钙动力学 [34].[ Albasher111 et al. further]。阿尔巴舍等人。进一步证明 demonstrated that Uro A
prevents streptozotocin通过激活 SIRT1 表达和脱乙酰酶活性来预防大鼠中链脲佐菌素 (STZ)
-induced DCM in 诱导的 DCM [ rats112 by activating SIRT1 expression and deacetylase activity [35].]。肖等人。表明 Xiao et al. suggested that Uro A
can attenuate DM-related cognitive impairment可以通过 N-聚糖生物合成途径改善全身炎症和肠道屏障功能障碍,从而减轻 DM 相关的认知障碍 [ by113]。这一结论也得到了 ameliorating systemic inflammation and intestinal barrier dysfunction through the N-glycan biosynthesis pathway [36]. This conclusion was also supported by Lee
et al. They pointed out that 等人的支持。他们指出,Uro A
prevented DM-associated AD by reducing transglutaminase type 2 通过减少 2 型转谷氨酰胺酶 (TGM2)
-dependent mitochondria-associated ER membrane 依赖性线粒体相关 ER 膜 (MAM)
formation and maintaining mitochondrial calcium and ROS homeostasis的形成和维持线粒体钙和 ROS 稳态来预防 [37].DM Xu相关的 et al.AD [ indicated35 that]。徐等人。表明 Uro A
ameliorated diabetic retinopathy by activating the 通过激活 Nrf2/HO-1
pathway to通路抑制炎症和氧化应激来改善糖尿病视网膜病变 [ inhibit114 inflammation]。周等人。发现余甘子通过调节由 and oxidative stress [38]. Zhou et al. found that Phyllanthus emblica L. facilitated vascular function in SET
Z-induced hyperglycemia rats by regulating 代谢物介导的 Akt/β-
catenin signaling, mediated by the ETs metabolites连环蛋白信号传导促进 STZ 诱导的高血糖大鼠的血管功能 [39].
Insulin[ resistance115 is one of the core mechanisms of ]。
胰岛素抵抗是DM
. However, as a complex systemic metabolic disease, insulin resistance alone is not enough to cause DM. Islet dysfunction caused by the decrease in the total amount of islet 的核心机制之一。然而,作为一种复杂的全身性代谢疾病,仅靠胰岛素抵抗是不足以引起糖尿病的。胰岛β
cells is also the key to the pathogenesis of DM. Studies have shown that β cells in T2DM can be divided细胞总量减少引起的胰岛功能障碍也是DM发病的关键。研究表明,T2DM 中的 β 细胞可分为三种主要状态:易感性、适应和失效[ into three main states: susceptibility, adaptation, and failure [40][41][42]. During insulin resistance, 116、117、118 ]。在胰岛素抵抗期间,β
cells细胞通过分泌胰岛素增加胰岛素需求来补偿功能障碍 compensate[ for90 the]。当 dysfunction by increasing insulin demand through insulin secretion [8]. When β
cells细胞不能补偿葡萄糖稳态时,就会发生高血糖症。更重要的是,来自余甘子的 fail to compensate for glucose homeostasis, hyperglycemia occurs. More importantly, EA
from Phyllanthus emblica L.
increased the size or增加糖尿病大鼠 number of β cells in diabetic rats. EA also directly increased glucose-stimulated insulin secretion from isolated islets, suggesting that EA acted directly on pancreatic β cells toβ 细胞的大小或数量。EA 还直接增加分离胰岛的葡萄糖刺激的胰岛素分泌,这表明 EA 直接作用于胰腺 β 细胞以发挥抗糖尿病活性,从而刺激胰岛素分泌并减少葡萄糖耐受不良 [ exert93 anti-diabetic activity, thereby stimulating insulin secretion and reducing glucose intolerance [11]. Histopathological results showed that ]。组织病理学结果表明,Uro A
had protective effects对 on β cells, such as improving the pancreatic structure and increasing islet size and number. Ultrastructural damages in DM mice pancreas after Uro A treatment, including ER expansion, mitochondriaβ 细胞具有保护作用,例如改善胰腺结构和增加胰岛大小和数量。Uro A 治疗后 DM 小鼠胰腺的超微结构损伤,包括 ER 扩张、线粒体肿胀、嵴骨折和髓鞘形成,也得到了显着改善 [ swelling, c90]。我们之前还讨论过,Ur
istae fracture, and myelin sheath fo
rmation, were also significantly improved [8]. A 通过激活自噬和调节 AKT/mT
heOR researchers also discussed earlier that Uro A prevented β-cell apoptosis in T2DM model mice by activating信号来预防 T2DM 模型小鼠的 β 细胞凋亡 [ autophagy88 and, regulating90 the AKT/mTOR signal [6][8][24]. However,
the102 specific mechanisms of ]。然而,Uro A
improving β-cell structure and function to mitigate DM risk改善 β 细胞结构和功能以降低 DM 风险的具体机制需要进一步探索。
总之,本节广泛讨论了 need to be explored further.
In summary, the metabolism and the roles of Uro A in ameliorating pancreatic diseases have been extensively discussed in this section (summarized in Figure在改善胰腺疾病中的代谢和作用(总结在图 1).2中)。通过阐明 By clarifying Uro A’s metabolism in vivo and Uro A’s mechanisms for protecting the pancreas, it might shed new light on managing pancreatic injuries via plant-based foods rich in ETs and EA.
的体内代谢和 Uro A 保护胰腺的机制,它可能为通过富含 ET 和 EA 的植物性食物管理胰腺损伤提供新的思路。