Phlorotannins (PTs), an important group of algae-derived polyphenolic compounds, have been considered potent antibacterial agents both as single drug entities and in combination with commercially available antibacterial drugs.
- brown algae
- marine algae
- antibacterial activity
- polyphenols
- phlorotannin
- antioxidant
- antibiotic
1. Introduction
2. Structural Diversity of Phlorotannins
Marine algae are a very rich source of secondary metabolites with a variety of chemical structures and, correspondingly, a wide range of biological properties [11,43,44,45][11][33][34][35]. Marine brown seaweeds contain, among others, phloroglucinol and its polymers, such as PTs [46,47][36][37]. Nevertheless, red algae also contain 1.8–3.2% of PTs [48][38]. PTs are a type of tannin primarily found in brown algae, such as kelps, rockweeds, and sargassacean species, but also in small amounts in red algae. Phlorotannins are a special group of hydrophilic phenolic compounds that display strong binding efficacy to polysaccharides, proteins, biopolymers, and other chelate divalent metals. They exhibit a huge variety of chemical structures and, consequently, polymeric properties [49,50,51][39][40][41]. They resemble tannins from terrestrial plants and are thought to play a role in cell wall formation. Phlorotannins are made by polymerizing phloroglucinol (1,3,5-trihydroxybenzene) monomer units in a range of combinations, similarly to the well-studied biosynthesis of terrestrial plant tannins from alcoholic monomers [52,53,54][42][43][44]. However, the biochemical mechanism for phlorotannin biosynthesis is poorly understood, with several hypotheses ranging from acetate and malonate unit condensation to the shikimate or phenylpropanoid pathways. PTs can also be found in a sulfated or halogenated state and their biosynthesis is carried out in the Golgi apparatus of the cell via an acetate-malonate pathway [55][45]. Glombitza was the first to develop a nomenclature scheme for marine phlorotannins according to the type of linkages and arrangement of the phloroglucinol monomers, the presence of an additional hydroxyl group for fuhalols, the existence of carmalols, and the potential presence of halogens, or sulfate groups [54,56][44][46]. PTs are generally divided into four main subclasses, i.e., phlorethols and fuhalols when joined through ether bonds, fucols when joined by phenyl bonds, fucophlorethols when they have both ether and phenyl bonds, and phloreckols when they contain dibenzodioxin bonds (Figure 1).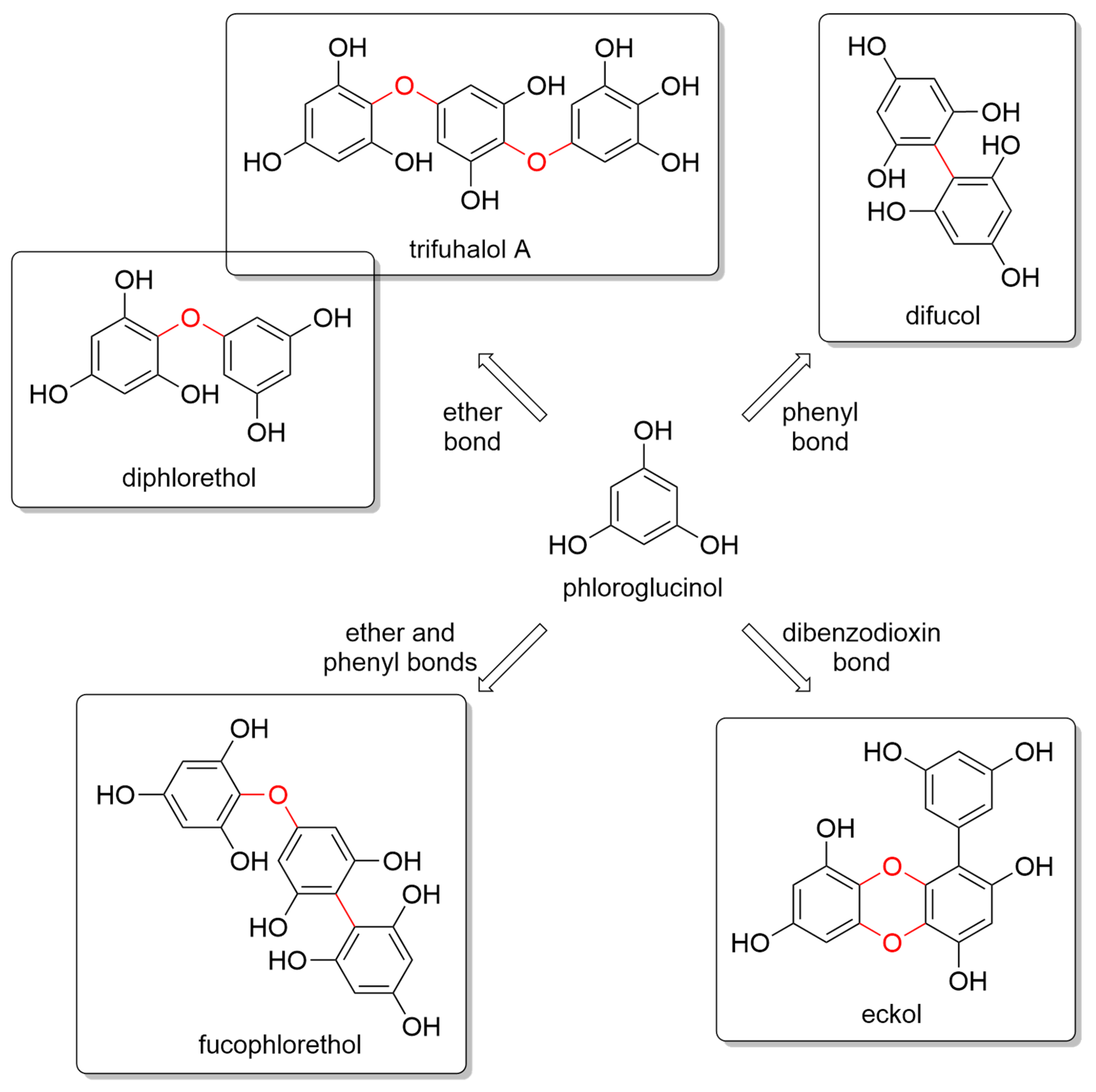
3. Extraction Procedure of Polyphenols from Marine Algae
The extraction of polyphenols from seaweed is generally carried out by using polar organic solvents, such as ethanol, methanol, and acetone [39,48,64,65,66][38][54][55][56][57]. The most common solvents used for the extraction of PTs are aqueous solutions of acetone or ethanol [63,67][53][58]. Brown algae produce a wide range of polymers, but their physiological activity is unknown. To extract PTs, the optimal temperature must not exceed 52 °C as higher temperatures can lead to their degradation [48][38]. Because of the low selectivity of the target component, of long extraction times, and the necessity to purify further the extract, solid-liquid extraction procedures are commonly utilized for isolating PTs from algae [64][55]. Polyphenols from Eisenia bicyclis, as well as other forms of brown and red algae, were extracted with both distilled water and a mixture of methanol, water, and acetic acid (30:69:1 v/v/v) to obtain a significant quantity of polyphenols (about 193 mg/g gallic acid equivalents, GAE). Extraction by 80% methanol yielded the highest amount of polyphenols (about 15 mg/g GAE) from Laminaria japonica, whereas extraction with 100% methanol gave the highest yield of polyphenols (over 8 mg/g GAE) from Undaria pinnatifida [48][38]. The extraction of polyphenols from Fucus evanescens was higher when an aqueous solution of ethanol was employed as a solvent, while distilled water was utilized for the extraction of polyphenols from S. japonica and Anfeltia tobuchiensis [68][59]. Many previous works claim that extraction with methanol exhibited the highest yield of PTs [65,66][56][57]. Ethyl acetate also extracted high amount of PTs from Sargassum fusiforme (almost 90 mg phloroglucinol equivalents/100 mg of extract) [69][60]. Nevertheless, acetone also allowed for excellent extraction of PTs. With traditional solvent-based methods of extraction, high-molecular weight PTs associated with the cell wall are not isolated [65,68][56][59]. Conversely, effective techniques for extracting polyphenols involve ultrasonication, enzymatic extraction, microwave, liquid extraction under pressure, and supercritical fluid extraction [64,66,68,70][55][57][59][61]. Among them, enzymatic extraction is very effective allowing algal cell wall destruction and high PTs recovery (21–38%) compared to solid-liquid extraction (3–15%) [66][57]. Mass transfer is stimulated during ultrasonic extraction by breaking plant cell walls, which enhances the release of high molecular weight PTs [64][55]. Microwave extraction has the benefit of yielding high amounts of polyphenols from plants while lowering extraction time and solvent use [64,66,68][55][57][59]. The time required to recover polyphenols is also greatly reduced when using high-pressure liquid extraction [39,48,64][38][54][55]. A considerable number of extraction methods have been employed for the extraction of PTs from algae, usually followed by chromatographic techniques for their purification [66][57]. To identify, quantify, and perform a structural analysis of PTs, nuclear magnetic resonance (NMR) spectroscopy and chromatography-mass spectrometry are generally used [70][61]. The existence of polysaccharide complexes as the principal component of the algal cell wall represents a substantial obstacle in polyphenols extraction, as PTs are included in the cell wall and are covalently bound to polysaccharides and proteins [71][62]. Nevertheless, modern chromatographic methods currently represent the state-of-the-art method for purifying and identifying PTs.4. Antioxidant Properties of Algal Phlorotannins
PTs are biologically active compounds with anti-inflammatory, anti-allergic, antiviral, antitumor, antioxidant, antidiabetic, and radioprotective effects [72,73,74,75][63][64][65][66]. PTs from algal sources act as electron traps for free radicals [76][67], and display robust antioxidant activity thanks to the numerous hydroxyl groups, thereby being toxic to bacteria under aerobic conditions [77][68]. Ethanol extracts of algae from the genera Agarum, Arthrothamnus, Fucus, Stephanocystis, and Thalassiophyllum, showed effective antioxidant properties. PTs from F. evanescens, Thalassiophyllum clathrus, and Stephanocystis crassipes also exhibited significant antioxidant activity [64,68][55][59]. In addition, PTs extracted from the brown alga Eisenia bicyclis displayed 10 times higher antioxidant activity over ascorbic acid. The antioxidant activity of PTs depends largely on the molecular weight of the compounds [51,78,79][41][69][70]. Algal phenolic, polyphenolic, and flavonoid molecules stimulate a wide range of biological functions. Numerous biochemical assays have been exploited to assess the ability of PTs to scavenge free radicals, such as the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical scavenging activity. When compared to ascorbic acid and α-tocopherol, PTs from brown algae E. cava, E. kurome, and E. bicyclics displayed considerable radical scavenging ability against the superoxide anion (Inhibitory Concentration–IC50–6.5–8.4 µM) and DPPH (IC50 12–26 µM) [79][70]. The antioxidant activity of diphlorethohydroxycarmalol from brown algae Ishige okamurae was determined using the DPPH assay and the IC50 value was found to be between 3.41 and 4.92 mM [80][71]. The IC50 of PT fractions from Sargassum ringgoldianum against superoxide anion radicals was evaluated to be 1.0 mg/mL, which was about five times stronger than catechin [81][72]. 974-A, 974-B, phlorofucofuroeckol-A, and dieckol had significantly lower IC50 values than phlorofucofuroeckol-B, phloroglucinol, α-tocopherol, and ascorbic acid [82][73]. To date, natural antioxidants are considered harmless for human beings. In this regard, PTs have the ability to scavenge ROS such as peroxyl, hydroxyl, and superoxide radicals [83][74]. The DPPH free radical scavenging of Sargassum aquifolium displayed a maximum of almost 7 mg phlorotannin per g of dry weight extract compared to the approximately 6 mg/g obtained with ascorbic acid [84][75]. Phenolic compounds and PTs extracted from brown seaweed, such as Trifucodiphlorethol, Trifucotriphlorethol, and Tucotriphlorethol, were shown to have IC50 values ranging between 10 and 14 mg/mL [85][76]. PTs isolated from E. cava also showed promising antioxidant capacity [86][77]. The information presented here could serve to better understand the biological properties of E. cava, other marine brown seaweeds, and their derivatives, as well as their potential use as functional ingredients in industrial applications.5. Mechanisms of Action of Phlorotannins Antibacterial Activity
PTs are the most effective agents for fighting bacterial biofilms because they penetrate the bacterial cell wall by changing the shape of the cell membrane and causing cell death [87,88][78][79]. Bacterial cell wall permeability is damaged by PTs, which cause proton leakage in the cell membrane, thus structural changes in the nuclear membrane leading to bacterial cell death [53,89,90][43][80][81]. In addition, PTs have the ability to eradicate bacteria by inhibiting their reproduction. The antibacterial activity of PTs has been attributed to their capacity for blocking oxidative phosphorylation, as well as to their ability to attach to bacterial proteins and enzymes, causing cell lysis. The phenolic aromatic rings and the -OH groups of phloroglucinol bind to the -NH groups of bacterial proteins, leading to inhibition [91,92][82][83]. The presence of additional groups, such as the two acetyl residues in 2,4-diacetylphloroglucinol (DAPG) or the 1-methylvinyl residue at the C-3 of ialibinones, can improve the bacteriolytic activity of phloroglucinol compounds [93,94][84][85]. Several investigations found that PTs play an important role in suppressing bacterial reproduction [90][81]. In addition, they bind to bacterial RNA and DNA, again inhibiting bacterial replication [57][47]. PTs are most effective against gram-negative bacteria as they can bind to the thick coating of the peptidoglycan and the lipopolysaccharides present in these bacteria [67][58]. When PTs bind to the cell wall of gram-negative bacteria, their permeability changes [87,95,96][78][86][87]. Moreover, PTs can modify the bacterial phosphotyrosine, thus inactivating the protein and the DNA replication, finally leading to bacterial growth inhibition [97][88]. Some studies reported that PTs harm bacteria’s cell walls by forming a pit outside the cell, thus interfering with bacterial functions, including permeability and respiration, reducing the cell’s reproductive capability, and eventually leading to cell death [87,95,96][78][86][87]. PTs might downregulate the activity of antioxidant enzymes such as SOD, CAT, and GSH, disturbing the redox-homeostasis and subsequently inducing ROS-mediated cell death. However, the exact mechanism is still poorly understood. Hence, research studies should also focus in this direction in order to draw a comprehensive conclusion. The overall mechanism of the ROS-mediated bacterial cell death by PTs is displayed in Figure 2.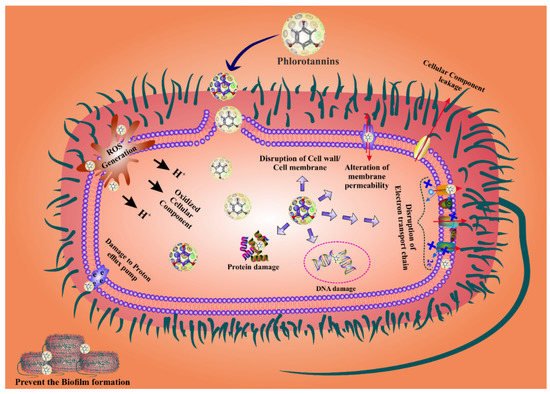
6. In Vitro Antibacterial Activity of Phlorotannins
Phlorotannins, and phenolic compounds in marine algae in general, have been shown to possess, among others, antibacterial activity, as summarized in Table 1.|
Phlorotannins |
Extract |
Bacteria |
Effect |
Ref. |
|---|---|---|---|---|
|
PTs aqueous extract |
Ericaria crinita (formerly known as Cystoseira crinita) |
Klebsiella, Bacillus cereus |
MIC of 25 mg/mL MIC of 25 mg/mL |
|
|
PTs ethyl acetate extract |
Ecklonia stolonifera and Ecklonia cava |
methicillin-resistant Staphylococcus aureus (MRSA) |
antibacterial efficacy |
|
|
Phlorofucofuroeckol-A |
E. bicyclics |
MRSA |
inhibited bacterial growth |
|
|
Low molecular weight PTs |
Sargassum thunbergia |
Vibrio parahaemolyticus |
cell membrane and cell wall damage, facilitating cytoplasm leakage and membrane permeability |
|
|
Phlorofucofuroeckol derivative |
E. bicyclics |
Propionibacterium |
MIC of 32 g/mL; reduced resistance to erythromycin and lincomycin |
|
|
Phlorofucofuroeckol |
Eisenia bicyclis |
MRSA |
inhibited expression of mecI, mecR1, and mecA genes and regulated expression of methicillin resistance by suppressing penicillin-binding protein 2a production |
|
|
Dieckol |
E. stolonifera |
MRSA |
synergistic effect with ampicillin (MIC from 512 to 0.5 mg/mL) |
|
|
Eckol |
E. cava |
S. aureus |
synergistic effect with ampicillin (eckol FIC from 0.3 to 0.5 µg/mL) |
|
|
PTs extract |
Ascophyllum nodosum |
E. coli |
inhibition of biofilm formation within 24 h of incubation |
|
|
PTs methanol extract |
Halidrys siliquosa |
S. aureus |
MIC and MBC from 0.1562 to 0.3125 mg/mL |
|
|
PTs-rich extract |
A. nodosum |
Porphyromonas gingivalis |
significantly reduced secretion of inflammatory cytokines and lowered lipid peroxidation |
References
- dos Santos Amorim, R.D.N.; Gurcel Rodrigues, J.A.; Holanda, M.L.; Gomes Quinderé, A.L.; Monteiro de Paula, R.C.; Maciel Melo, V.M.; Barros Benevides, N.M. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornata. Braz. Arch. Biol. Technol. 2012, 55, 171–181.
- Malhotra, S.; Singh, A. Algae, traditional medicine, and pharmacological advances. Int. J. Algae 2008, 10, 299–308.
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. 2021, 30, e00633.
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomed. Int. J. Phytother. Phytopharm. 2021, 90, 153554.
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Panda, K.C.; Das, S.; Jena, M. Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: Current evidences and future perspectives. Phytother. Res. 2021, 35, 4194–4214.
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Proc. Semin. Cancer Biol. 2021, 73, 310–320.
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.S.; Jena, M. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242.
- Jit, B.P.; Pradhan, B.; Dash, R.; Bhuyan, P.P.; Behera, C.; Behera, R.K.; Sharma, A.; Alcaraz, M.; Jena, M. Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways. Antioxidants 2022, 11, 49.
- Jit, B.P.; Pattnaik, S.; Arya, R.; Dash, R.; Sahoo, S.S.; Pradhan, B.; Bhuyan, P.P.; Behera, P.K.; Jena, M.; Sharma, A.; et al. Phytochemicals: A potential next generation agent for radioprotection. Phytomed. Int. J. Phytother. Phytopharm. 2022, 154188.
- Quarta, A.; Gaballo, A.; Pradhan, B.; Patra, S.; Jena, M.; Ragusa, A. Beneficial Oxidative Stress-Related trans-Resveratrol Effects in the Treatment and Prevention of Breast Cancer. Appl. Sci. 2021, 11, 11041.
- Pradhan, B.; Kim, H.; Abassi, S.; Ki, J.-S. Toxic Effects and Tumor Promotion Activity of Marine Phytoplankton Toxins: A Review. Toxins 2022, 14, 397.
- Pradhan, B.; Ki, J.-S. Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals. Mar. Drugs 2022, 20, 271.
- Tagliabue, A.; Rappuoli, R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018, 9, 1068.
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224.
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9, 200.
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Marine algae as efficacious bioresources housing antimicrobial compounds for preserving foods—A review. Int. J. Food Microbiol. 2021, 358, 109416.
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262.
- Maharana, S.; Pradhan, B.; Jena, M.; Misra, M.K. Diversity of Phytoplankton in Chilika Lagoon, Odisha, India. Environ. Ecol. 2019, 37, 737–746.
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91.
- Behera, C.; Pradhan, B.; Panda, R.; Nayak, R.; Nayak, S.; Jena, M. Algal Diversity of Saltpans, Huma (Ganjam), India. J. Indian Bot. Soc. 2021, 101, 107–120.
- Pradhan, B.; Maharana, S.; Bhakta, S.; Jena, M. Marine phytoplankton diversity of Odisha coast, India with special reference to new record of diatoms and dinoflagellates. Vegetos 2021, 35, 330–344.
- Behera, C.; Dash, S.R.; Pradhan, B.; Jena, M.; Adhikary, S.P. Algal diversity of Ansupa lake, Odisha, India. Nelumbo 2020, 62, 207–220.
- Dash, S.; Pradhan, B.; Behera, C.; Nayak, R.; Jena, M. Algal Flora of Tampara Lake, Chhatrapur, Odisha, India. J. Indian Bot. Soc. 2021, 101, 1–15.
- Dash, S.; Pradhan, B.; Behera, C. Algal Diversity of Kanjiahata Lake, Nandankanan, Odisha, India. J. Indian Bot. Soc. 2020, 99, 11–24.
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Patil, S.; Bhutia, S.K.; Jena, M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in oral cancer. Mol. Biol. Rep. 2020, 47, 9567–9578.
- Mohanty, S.; Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jena, M. Screening for nutritive bioactive compounds in some algal strains isolated from coastal Odisha. J. Adv. Plant Sci. 2020, 10, 1–8.
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.; Ragusa, A.; Jena, M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2021, 26, 37.
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579.
- Stabili, L.; Acquaviva, M.I.; Angilè, F.; Cavallo, R.A.; Cecere, E.; Del Coco, L.; Fanizzi, F.P.; Gerardi, C.; Narracci, M.; Petrocelli, A. Screening of Chaetomorpha linum Lipidic Extract as A New Potential Source of Bioactive Compounds. Mar. Drugs 2019, 17, 313.
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Phenolic composition, antioxidant activity, anticholinesterase potential and modulatory effects of aqueous extracts of some seaweeds on β-amyloid aggregation and disaggregation. Pharm. Biol. 2019, 57, 460–469.
- Narasimhan, M.K.; Pavithra, S.K.; Krishnan, V.; Chandrasekaran, M. In vitro Analysis of Antioxidant, Antimicrobial and Antiproliferative Activity of Enteromorpha antenna, Enteromorpha linza and Gracilaria corticata Extracts. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 151–159.
- Chakraborty, K.; Maneesh, A.; Makkar, F. Antioxidant activity of brown seaweeds. J. Aquat. Food Prod. 2017, 26, 406–419.
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Dash, S.R.; Ki, J.-S.; Adhikary, S.P.; Ragusa, A.; Jena, M. Cyanobacteria and Algae-Derived Bioactive Metabolites as Antiviral Agents: Evidence, Mode of Action, and Scope for Further Expansion; A Comprehensive Review in Light of the SARS-CoV-2 Outbreak. Antioxidants 2022, 11, 354.
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Behera, P.K.; Mandal, A.K.; Behera, C.; Ki, J.-S.; Adhikary, S.P.; MubarakAli, D.; et al. A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections. Carbohydr. Polym. 2022, 291, 119551.
- Shrestha, S.; Zhang, W.; Smid, S. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832.
- van Alstyne, K.L. Comparison of three methods for quantifying brown algal polyphenolic compounds. J. Chem. Ecol. 1995, 21, 45–58.
- Poole, J.; Diop, A.; Rainville, L.-C.; Barnabé, S. Bioextracting Polyphenols from the Brown Seaweed Ascophyllum nodosum from Québec’s North Shore Coastline. Ind. Biotechnol. 2019, 15, 212–218.
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133.
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2007, 18, 326–332.
- Boi, V.N.; Trang, N.T.M.; Cuong, D.X.; Ha, H.T. Antioxidant Phlorotannin from Brown Algae Sargassum dupplicatum: Enzyme-assissted Extraction and Purification. World 2020, 4, 62–68.
- Wang, T.; Jόnsdόttir, R.S.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdόttir, G.N. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883.
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine Algal Derived Phenolic Compounds and their Biological Activities for Medicinal and Cosmetic Applications. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; Rajauria, G., Yuan, Y.V., Eds.; Wiley-VCH: Weinheim, Germany, 2021; pp. 278–334.
- Cabral, E.M.; Oliveira, M.; Mondala, J.R.; Curtin, J.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobials from Seaweeds for Food Applications. Mar. Drugs 2021, 19, 211.
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/function analysis of a type iii polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 2013, 25, 3089–3103.
- Moon, C.; Kim, S.H.; Kim, J.C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother. Res. 2008, 22, 238–242.
- Glombitza, K.W. Marine Natural Product Chemistry; Faulkner, D.J., Fenical, W.H., Eds.; Plenum Press: New York, NY, USA, 1977.
- Manandhar, B.; Paudel, P. Characterizing Eckol as a Therapeutic Aid: A Systematic Review. Mar. Drugs 2019, 17, 361.
- Lüder, U.H.; Clayton, M.N. Induction of phlorotannins in the brown macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to simulated herbivory—The first microscopic study. Planta 2004, 218, 928–937.
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614.
- Jegan, S.; Raj, G.A.; Chandrasekaran, M.; Venkatesalu, V. Anti-MRSA activity of Padina tetrastromatica, Padina gymnospora from Gulf of Mannar biosphere. World Sci. News 2019, 115, 15–26.
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681.
- Kim, S.M.; Kang, S.W.; Jeon, J.S.; Jung, Y.J.; Kim, W.R.; Kim, C.Y.; Um, B.H. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406.
- Schoenwaelder, M.E.A.; Clayton, M.N. The presence of phenolic compounds in isolated cell walls of brown algae. Phycologia 1999, 38, 161–166.
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273.
- Aminina, N.M.; Vishnevskaya, T.I.; Karaulova, E.P.; Epur, N.V.; Yakush, E.V. Prospects for the use of commercial and potentially commercial brown algae of the Far Eastern seas as a source of polyphenols. Russ. J. Mar. Biol. 2020, 46, 34–41.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901.
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total phenolic content and biological activities of enzymatic extracts from Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537.
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145.
- Aminina, N.; Vishnevskaya, T.; Karaulova, E.; Yakush, E. Content of polyphenols and antioxidant activity of extracts from certain species of seaweeds. Izv. TINRO 2017, 189, 184–191.
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49.
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6.
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250.
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489.
- Li, Y.; Lee, S.H.; Le, Q.T.; Kim, M.M.; Kim, S.K. Anti-allergic Effects of Phlorotannins on Histamine Release via Binding Inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080.
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.W.; Carmeli, S.; Klimo, K.; Gerhäuser, C.; König, G.M. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2010, 71, 221–229.
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408–414.
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609.
- Kim, S.-K.; Chojnacka, K. Marine Algae Extracts: Processes, Products, and Applications (2-Volume Set); Kim, S.-K., Chojnacka, K., Eds.; Wiley-VCH: Weinheim, Germany, 2015.
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Gall, E.A. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405.
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705.
- Heo, S.J.; Kim, J.P.; Jung, W.K.; Lee, N.H.; Kang, H.S.; Jun, E.M.; Park, S.H.; Kang, S.M.; Lee, Y.J.; Park, P.J.; et al. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol. 2008, 18, 676–681.
- Nakai, M.; Kageyama, N.; Nakahara, K.; Miki, W. Phlorotannins as radical scavengers from the extract of Sargassum ringgoldianum. Mar. Biotechnol. 2006, 8, 409–414.
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and structural determination of two novel phlorotannins from the brown alga Ecklonia kurome Okamura, and their radical scavenging activities. Mar. Drugs 2013, 11, 165–183.
- Kirke, D.A.; Smyth, T.J.; Rai, D.K.; Kenny, O.; Stengel, D.B. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2017, 221, 1104–1112.
- Cuong, D.X.; Boi, V.N.; Van, T.T.T.; Hau, L.N. Effect of storage time on phlorotannin content and antioxidant activity of six Sargassum species from Nhatrang Bay, Vietnam. J. Appl. Phycol. 2016, 28, 567–572.
- Parys, S.; Rosenbaum, A.; Kehraus, S.; Reher, G.; Glombitza, K.W.; König, G.M. Evaluation of quantitative methods for the determination of polyphenols in algal extracts. J. Nat. Prod. 2007, 70, 1865–1870.
- Le, Q.-T.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176.
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81.
- López, Y.; Cepas, V.; Soto, S.M. The Marine Ecosystem as a Source of Antibiotics. In Grand Challenges in Marine Biotechnology; Rampelotto, P., Trincone, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–48.
- Ramadan, G.; Fouda, W.A.; Ellamie, A.M.; Ibrahim, W.M. Dietary supplementation of Sargassum latifolium modulates thermo-respiratory response, inflammation, and oxidative stress in bacterial endotoxin-challenged male Barki sheep. Environ. Sci. Pollut. Res. Int. 2020, 27, 33863–33871.
- Potin, P.; Bouarab, K.; Salaün, J.-P.; Pohnert, G.; Kloareg, B. Biotic interactions of marine algae. Curr. Opin. Plant Biol. 2002, 5, 308–317.
- Piechulla, B.; Heldt, H.-W. Plant Biochemistry, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2011.
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia coli to Seaweed (Ascophyllum nodosum) Phlorotannins and Terrestrial Tannins. Asian-Aust. J. Anim. Sci. 2009, 22, 238–245.
- Kamei, Y.; Isnansetyo, A. Lysis of methicillin-resistant Staphylococcus aureus by 2,4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga. Int. J. Antimicrob. Agents 2003, 21, 71–74.
- Winkelman, K.; Heilman, J.; Zerbe, O.; Rali, T.; Sticher, O. New phlorolucinol derivates from Hyperium papuanum. J. Nat. Prod. 2000, 63, 104–108.
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342.
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103.
- Peng, S.; Zhao, M. Pharmaceutical Bioassays: Methods and Applications; Wiley-VCH: Weinheim, Germany, 2009.
- Eom, S.-H.; Lee, D.-S.; Jung, Y.-J.; Park, J.-H.; Choi, J.-I.; Yim, M.-J.; Jeon, J.-M.; Kim, H.-W.; Son, K.-T.; Je, J.-Y. The mechanism of antibacterial activity of phlorofucofuroeckol-A against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804.
- Eom, S.H.; Kim, D.H.; Lee, S.H.; Yoon, N.Y.; Kim, J.H.; Kim, T.H.; Chung, Y.H.; Kim, S.B.; Kim, Y.M.; Kim, H.W. In vitro antibacterial activity and synergistic antibiotic effects of phlorotannins isolated from Eisenia bicyclis against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2013, 27, 1260–1264.
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255.
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the membrane permeability and cell death of Vibrio parahaemolyticus caused by phlorotannins with low molecular weight from Sargassum thunbergii. J. Aquat. Food Prod. 2016, 25, 323–333.
- Lee, J.-H.; Eom, S.-H.; Lee, E.-H.; Jung, Y.-J.; Kim, H.-J.; Jo, M.-R.; Son, K.-T.; Lee, H.-J.; Kim, J.H.; Lee, M.-S. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55.
- Lee, S.H.; Kim, S.K. Biological Phlorotannins of Eisenia bicyclis. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S.-K., Chojnacka, K., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 453–464.
- Lee, D.-S.; Kang, M.-S.; Hwang, H.-J.; Eom, S.-H.; Yang, J.-Y.; Lee, M.-S.; Lee, W.-J.; Jeon, Y.-J.; Choi, J.-S.; Kim, Y.-M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioproc. E 2008, 13, 758–764.
- Oliver, S.P. Foodborne Pathogens and Disease Special Issue on the National and International PulseNet Network. Foodborne Pathog. Dis. 2019, 16, 439–440.
- Busetti, A.; Thompson, T.P.; Tegazzini, D.; Megaw, J.; Maggs, C.A.; Gilmore, B.F. Antibiofilm Activity of the Brown Alga Halidrys siliquosa against Clinically Relevant Human Pathogens. Mar. Drugs 2015, 13, 3581–3605.
- Tamanai-Shacoori, Z.; Chandad, F.; Rébillard, A.; Cillard, J.; Bonnaure-Mallet, M. Silver-zeolite combined to polyphenol-rich extracts of Ascophyllum nodosum: Potential active role in prevention of periodontal diseases. PLoS ONE 2014, 9, e105475.
