Phlorotannins (PTs), an important group of algae-derived polyphenolic compounds, have been considered potent antibacterial agents both as single drug entities and in combination with commercially available antibacterial drugs.
- brown algae
- marine algae
- antibacterial activity
- polyphenols
- phlorotannin
- antioxidant
- antibiotic
1. Introduction
2. Structural Diversity of Phlorotannins
Marine algae are a very rich source of secondary metabolites with a variety of chemical structures and, correspondingly, a wide range of biological properties [11][33][34][35][11,43,44,45]. Marine brown seaweeds contain, among others, phloroglucinol and its polymers, such as PTs [36][37][46,47]. Nevertheless, red algae also contain 1.8–3.2% of PTs [38][48]. PTs are a type of tannin primarily found in brown algae, such as kelps, rockweeds, and sargassacean species, but also in small amounts in red algae. Phlorotannins are a special group of hydrophilic phenolic compounds that display strong binding efficacy to polysaccharides, proteins, biopolymers, and other chelate divalent metals. They exhibit a huge variety of chemical structures and, consequently, polymeric properties [39][40][41][49,50,51]. They resemble tannins from terrestrial plants and are thought to play a role in cell wall formation. Phlorotannins are made by polymerizing phloroglucinol (1,3,5-trihydroxybenzene) monomer units in a range of combinations, similarly to the well-studied biosynthesis of terrestrial plant tannins from alcoholic monomers [42][43][44][52,53,54]. However, the biochemical mechanism for phlorotannin biosynthesis is poorly understood, with several hypotheses ranging from acetate and malonate unit condensation to the shikimate or phenylpropanoid pathways. PTs can also be found in a sulfated or halogenated state and their biosynthesis is carried out in the Golgi apparatus of the cell via an acetate-malonate pathway [45][55]. Glombitza was the first to develop a nomenclature scheme for marine phlorotannins according to the type of linkages and arrangement of the phloroglucinol monomers, the presence of an additional hydroxyl group for fuhalols, the existence of carmalols, and the potential presence of halogens, or sulfate groups [44][46][54,56]. PTs are generally divided into four main subclasses, i.e., phlorethols and fuhalols when joined through ether bonds, fucols when joined by phenyl bonds, fucophlorethols when they have both ether and phenyl bonds, and phloreckols when they contain dibenzodioxin bonds (Figure 1).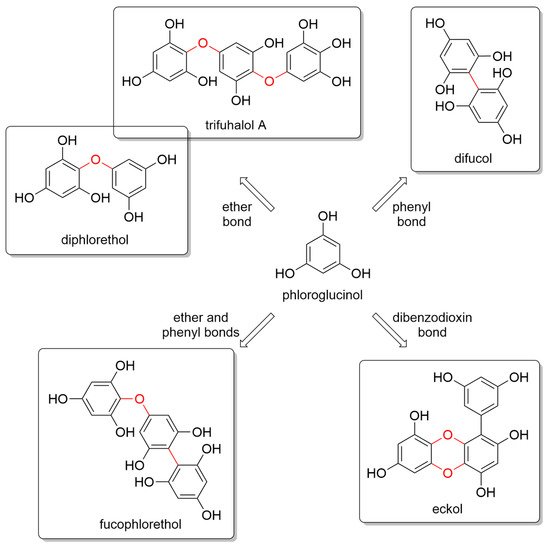
3. Extraction Procedure of Polyphenols from Marine Algae
The extraction of polyphenols from seaweed is generally carried out by using polar organic solvents, such as ethanol, methanol, and acetone [38][54][55][56][57][39,48,64,65,66]. The most common solvents used for the extraction of PTs are aqueous solutions of acetone or ethanol [53][58][63,67]. Brown algae produce a wide range of polymers, but their physiological activity is unknown. To extract PTs, the optimal temperature must not exceed 52 °C as higher temperatures can lead to their degradation [38][48]. Because of the low selectivity of the target component, of long extraction times, and the necessity to purify further the extract, solid-liquid extraction procedures are commonly utilized for isolating PTs from algae [55][64]. Polyphenols from Eisenia bicyclis, as well as other forms of brown and red algae, were extracted with both distilled water and a mixture of methanol, water, and acetic acid (30:69:1 v/v/v) to obtain a significant quantity of polyphenols (about 193 mg/g gallic acid equivalents, GAE). Extraction by 80% methanol yielded the highest amount of polyphenols (about 15 mg/g GAE) from Laminaria japonica, whereas extraction with 100% methanol gave the highest yield of polyphenols (over 8 mg/g GAE) from Undaria pinnatifida [38][48]. The extraction of polyphenols from Fucus evanescens was higher when an aqueous solution of ethanol was employed as a solvent, while distilled water was utilized for the extraction of polyphenols from S. japonica and Anfeltia tobuchiensis [59][68]. Many previous works claim that extraction with methanol exhibited the highest yield of PTs [56][57][65,66]. Ethyl acetate also extracted high amount of PTs from Sargassum fusiforme (almost 90 mg phloroglucinol equivalents/100 mg of extract) [60][69]. Nevertheless, acetone also allowed for excellent extraction of PTs. With traditional solvent-based methods of extraction, high-molecular weight PTs associated with the cell wall are not isolated [56][59][65,68]. Conversely, effective techniques for extracting polyphenols involve ultrasonication, enzymatic extraction, microwave, liquid extraction under pressure, and supercritical fluid extraction [55][57][59][61][64,66,68,70]. Among them, enzymatic extraction is very effective allowing algal cell wall destruction and high PTs recovery (21–38%) compared to solid-liquid extraction (3–15%) [57][66]. Mass transfer is stimulated during ultrasonic extraction by breaking plant cell walls, which enhances the release of high molecular weight PTs [55][64]. Microwave extraction has the benefit of yielding high amounts of polyphenols from plants while lowering extraction time and solvent use [55][57][59][64,66,68]. The time required to recover polyphenols is also greatly reduced when using high-pressure liquid extraction [38][54][55][39,48,64]. A considerable number of extraction methods have been employed for the extraction of PTs from algae, usually followed by chromatographic techniques for their purification [57][66]. To identify, quantify, and perform a structural analysis of PTs, nuclear magnetic resonance (NMR) spectroscopy and chromatography-mass spectrometry are generally used [61][70]. The existence of polysaccharide complexes as the principal component of the algal cell wall represents a substantial obstacle in polyphenols extraction, as PTs are included in the cell wall and are covalently bound to polysaccharides and proteins [62][71]. Nevertheless, modern chromatographic methods currently represent the state-of-the-art method for purifying and identifying PTs.4. Antioxidant Properties of Algal Phlorotannins
PTs are biologically active compounds with anti-inflammatory, anti-allergic, antiviral, antitumor, antioxidant, antidiabetic, and radioprotective effects [63][64][65][66][72,73,74,75]. PTs from algal sources act as electron traps for free radicals [67][76], and display robust antioxidant activity thanks to the numerous hydroxyl groups, thereby being toxic to bacteria under aerobic conditions [68][77]. Ethanol extracts of algae from the genera Agarum, Arthrothamnus, Fucus, Stephanocystis, and Thalassiophyllum, showed effective antioxidant properties. PTs from F. evanescens, Thalassiophyllum clathrus, and Stephanocystis crassipes also exhibited significant antioxidant activity [55][59][64,68]. In addition, PTs extracted from the brown alga Eisenia bicyclis displayed 10 times higher antioxidant activity over ascorbic acid. The antioxidant activity of PTs depends largely on the molecular weight of the compounds [41][69][70][51,78,79]. Algal phenolic, polyphenolic, and flavonoid molecules stimulate a wide range of biological functions. Numerous biochemical assays have been exploited to assess the ability of PTs to scavenge free radicals, such as the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical scavenging activity. When compared to ascorbic acid and α-tocopherol, PTs from brown algae E. cava, E. kurome, and E. bicyclics displayed considerable radical scavenging ability against the superoxide anion (Inhibitory Concentration–IC50–6.5–8.4 µM) and DPPH (IC50 12–26 µM) [70][79]. The antioxidant activity of diphlorethohydroxycarmalol from brown algae Ishige okamurae was determined using the DPPH assay and the IC50 value was found to be between 3.41 and 4.92 mM [71][80]. The IC50 of PT fractions from Sargassum ringgoldianum against superoxide anion radicals was evaluated to be 1.0 mg/mL, which was about five times stronger than catechin [72][81]. 974-A, 974-B, phlorofucofuroeckol-A, and dieckol had significantly lower IC50 values than phlorofucofuroeckol-B, phloroglucinol, α-tocopherol, and ascorbic acid [73][82]. To date, natural antioxidants are considered harmless for human beings. In this regard, PTs have the ability to scavenge ROS such as peroxyl, hydroxyl, and superoxide radicals [74][83]. The DPPH free radical scavenging of Sargassum aquifolium displayed a maximum of almost 7 mg phlorotannin per g of dry weight extract compared to the approximately 6 mg/g obtained with ascorbic acid [75][84]. Phenolic compounds and PTs extracted from brown seaweed, such as Trifucodiphlorethol, Trifucotriphlorethol, and Tucotriphlorethol, were shown to have IC50 values ranging between 10 and 14 mg/mL [76][85]. PTs isolated from E. cava also showed promising antioxidant capacity [77][86]. The information presented here could serve to better understand the biological properties of E. cava, other marine brown seaweeds, and their derivatives, as well as their potential use as functional ingredients in industrial applications.5. Mechanisms of Action of Phlorotannins Antibacterial Activity
PTs are the most effective agents for fighting bacterial biofilms because they penetrate the bacterial cell wall by changing the shape of the cell membrane and causing cell death [78][79][87,88]. Bacterial cell wall permeability is damaged by PTs, which cause proton leakage in the cell membrane, thus structural changes in the nuclear membrane leading to bacterial cell death [43][80][81][53,89,90]. In addition, PTs have the ability to eradicate bacteria by inhibiting their reproduction. The antibacterial activity of PTs has been attributed to their capacity for blocking oxidative phosphorylation, as well as to their ability to attach to bacterial proteins and enzymes, causing cell lysis. The phenolic aromatic rings and the -OH groups of phloroglucinol bind to the -NH groups of bacterial proteins, leading to inhibition [82][83][91,92]. The presence of additional groups, such as the two acetyl residues in 2,4-diacetylphloroglucinol (DAPG) or the 1-methylvinyl residue at the C-3 of ialibinones, can improve the bacteriolytic activity of phloroglucinol compounds [84][85][93,94]. Several investigations found that PTs play an important role in suppressing bacterial reproduction [81][90]. In addition, they bind to bacterial RNA and DNA, again inhibiting bacterial replication [47][57]. PTs are most effective against gram-negative bacteria as they can bind to the thick coating of the peptidoglycan and the lipopolysaccharides present in these bacteria [58][67]. When PTs bind to the cell wall of gram-negative bacteria, their permeability changes [78][86][87][87,95,96]. Moreover, PTs can modify the bacterial phosphotyrosine, thus inactivating the protein and the DNA replication, finally leading to bacterial growth inhibition [88][97]. Some studies reported that PTs harm bacteria’s cell walls by forming a pit outside the cell, thus interfering with bacterial functions, including permeability and respiration, reducing the cell’s reproductive capability, and eventually leading to cell death [78][86][87][87,95,96]. PTs might downregulate the activity of antioxidant enzymes such as SOD, CAT, and GSH, disturbing the redox-homeostasis and subsequently inducing ROS-mediated cell death. However, the exact mechanism is still poorly understood. Hence, research studies should also focus in this direction in order to draw a comprehensive conclusion. The overall mechanism of the ROS-mediated bacterial cell death by PTs is displayed in Figure 2.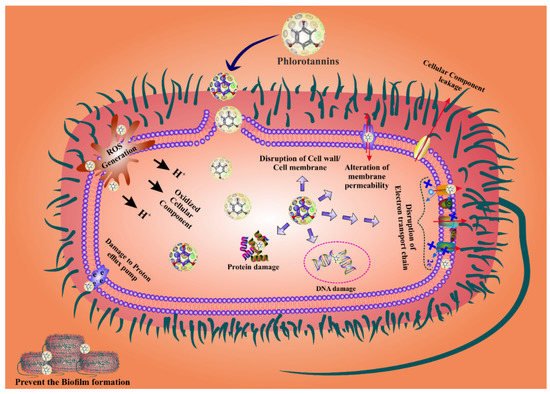
6. In Vitro Antibacterial Activity of Phlorotannins
Phlorotannins, and phenolic compounds in marine algae in general, have been shown to possess, among others, antibacterial activity, as summarized in Table 1.|
Phlorotannins |
Extract |
Bacteria |
Effect |
Ref. |
|---|---|---|---|---|
|
PTs aqueous extract |
Ericaria crinita (formerly known as Cystoseira crinita) |
Klebsiella, Bacillus cereus |
MIC of 25 mg/mL MIC of 25 mg/mL |
|
|
PTs ethyl acetate extract |
Ecklonia stolonifera and Ecklonia cava |
methicillin-resistant Staphylococcus aureus (MRSA) |
antibacterial efficacy |
|
|
Phlorofucofuroeckol-A |
E. bicyclics |
MRSA |
inhibited bacterial growth |
|
|
Low molecular weight PTs |
Sargassum thunbergia |
Vibrio parahaemolyticus |
cell membrane and cell wall damage, facilitating cytoplasm leakage and membrane permeability |
|
|
Phlorofucofuroeckol derivative |
E. bicyclics |
Propionibacterium |
MIC of 32 g/mL; reduced resistance to erythromycin and lincomycin |
|
|
Phlorofucofuroeckol |
Eisenia bicyclis |
MRSA |
inhibited expression of mecI, mecR1, and mecA genes and regulated expression of methicillin resistance by suppressing penicillin-binding protein 2a production |
|
|
Dieckol |
E. stolonifera |
MRSA |
synergistic effect with ampicillin (MIC from 512 to 0.5 mg/mL) |
|
|
Eckol |
E. cava |
S. aureus |
synergistic effect with ampicillin (eckol FIC from 0.3 to 0.5 µg/mL) |
|
|
PTs extract |
Ascophyllum nodosum |
E. coli |
inhibition of biofilm formation within 24 h of incubation |
|
|
PTs methanol extract |
Halidrys siliquosa |
S. aureus |
MIC and MBC from 0.1562 to 0.3125 mg/mL |
|
|
PTs-rich extract |
A. nodosum |
Porphyromonas gingivalis |
significantly reduced secretion of inflammatory cytokines and lowered lipid peroxidation |
